Photodynamic nasal SARS-CoV-2 decolonization shortens infectivity and influences specific T-Cell responses
- PMID: 36761900
- PMCID: PMC9905247
- DOI: 10.3389/fcimb.2023.1110467
Photodynamic nasal SARS-CoV-2 decolonization shortens infectivity and influences specific T-Cell responses
Abstract
Background: The main objective was to evaluate the efficacy of intranasal photodynamic therapy (PDT) in SARS-CoV-2 mildly symptomatic carriers on decreasing the infectivity period. SARS-CoV-2-specific immune-stimulating effects and safety were also analysed.
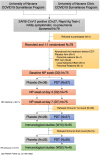
Methods: We performed a randomized, placebo-controlled, clinical trial in a tertiary hospital (NCT05184205). Patients with a positive SARS-CoV-2 PCR in the last 48 hours were recruited and aleatorily assigned to PDT or placebo. Patients with pneumonia were excluded. Participants and investigators were masked to group assignment. The primary outcome was the reduction in in vitro infectivity of nasopharyngeal samples at days 3 and 7. Additional outcomes included safety assessment and quantification of humoral and T-cell immune-responses.
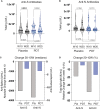
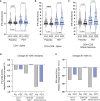
Findings: Patients were recruited between December 2021 and February 2022. Most were previously healthy adults vaccinated against COVID-19 and most carried Omicron variant. 38 patients were assigned to placebo and 37 to PDT. Intranasal PDT reduced infectivity at day 3 post-treatment when compared to placebo with a β-coefficient of -812.2 (CI95%= -478660 - -1.3, p<0.05) infectivity arbitrary units. The probability of becoming PCR negative (ct>34) at day 7 was higher on the PDT-group, with an OR of 0.15 (CI95%=0.04-0.58). There was a decay in anti-Spike titre and specific SARS-CoV-2 T cell immunity in the placebo group 10 and 20 weeks after infection, but not in the PDT-group. No serious adverse events were reported.
Interpretation: Intranasal-PDT is safe in pauci-symptomatic COVID-19 patients, it reduces SARS-CoV-2 infectivity and decelerates the decline SARS-CoV-2 specific immune-responses.
Keywords: COVID19; SARS-CoV-2; anti-spike antibody; infectivity; inmunity; photodisinfection; photodynamic therapy.
Copyright © 2023 Fernandez-Montero, Zuaznabar, Pina-Sanchez, Maestro, Martin-Navarro, Muñoz-Rodríguez, Olagüe, Pastrana, Martínez-Fernández, Camps, Rodriguez, Marchese, Zazpe, Pozuelo, Del Pozo, Quiroga, Pineda-Lucena, Reina, Kolenda, Moreno-Galarraga, Gonzalez-Aseguinolaza, Rua, Smerdou, Carmona-Torre and Argemi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Arentz J., von der Heide H. J. (2022). Evaluation of methylene blue based photodynamic inactivation (PDI) against intracellular b-CoV and SARS-CoV2 viruses under different light sources in vitro as a basis for new local treatment strategies in the early phase of a Covid19 infection. Photodiagnosis Photodyn. Ther. 37, 102642. doi: 10.1016/j.pdpdt.2021.102642 - DOI - PMC - PubMed
-
- Bouzid D., Visseaux B., Kassasseya C., Daoud A., Femy F., Hermand C., et al. . (2022). Comparison of patients infected with delta versus omicron COVID-19 variants presenting to Paris emergency departments: A retrospective cohort study. Ann. Intern. Med. 175 (6), 831–837. doi: 10.7326/M22-0308 - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

