The amyloid imaging for the prevention of Alzheimer's disease consortium: A European collaboration with global impact
- PMID: 36761917
- PMCID: PMC9907029
- DOI: 10.3389/fneur.2022.1063598
The amyloid imaging for the prevention of Alzheimer's disease consortium: A European collaboration with global impact
Abstract
Background: Amyloid-β (Aβ) accumulation is considered the earliest pathological change in Alzheimer's disease (AD). The Amyloid Imaging to Prevent Alzheimer's Disease (AMYPAD) consortium is a collaborative European framework across European Federation of Pharmaceutical Industries Associations (EFPIA), academic, and 'Small and Medium-sized enterprises' (SME) partners aiming to provide evidence on the clinical utility and cost-effectiveness of Positron Emission Tomography (PET) imaging in diagnostic work-up of AD and to support clinical trial design by developing optimal quantitative methodology in an early AD population.
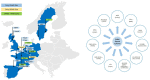
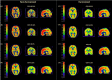
The amypad studies: In the Diagnostic and Patient Management Study (DPMS), 844 participants from eight centres across three clinical subgroups (245 subjective cognitive decline, 342 mild cognitive impairment, and 258 dementia) were included. The Prognostic and Natural History Study (PNHS) recruited pre-dementia subjects across 11 European parent cohorts (PCs). Approximately 1600 unique subjects with historical and prospective data were collected within this study. PET acquisition with [18F]flutemetamol or [18F]florbetaben radiotracers was performed and quantified using the Centiloid (CL) method.
Results: AMYPAD has significantly contributed to the AD field by furthering our understanding of amyloid deposition in the brain and the optimal methodology to measure this process. Main contributions so far include the validation of the dual-time window acquisition protocol to derive the fully quantitative non-displaceable binding potential (BP ND ), assess the value of this metric in the context of clinical trials, improve PET-sensitivity to emerging Aβ burden and utilize its available regional information, establish the quantitative accuracy of the Centiloid method across tracers and support implementation of quantitative amyloid-PET measures in the clinical routine.
Future steps: The AMYPAD consortium has succeeded in recruiting and following a large number of prospective subjects and setting up a collaborative framework to integrate data across European PCs. Efforts are currently ongoing in collaboration with ARIDHIA and ADDI to harmonize, integrate, and curate all available clinical data from the PNHS PCs, which will become openly accessible to the wider scientific community.
Keywords: Alzheimer's disease; amyloid; consortium; diagnosis; positron emission tomography (PET); prognosis.
Copyright © 2023 Collij, Farrar, Valléz García, Bader, Shekari, Lorenzini, Pemberton, Altomare, Pla, Loor, Markiewicz, Yaqub, Buckley, Frisoni, Nordberg, Payoux, Stephens, Gismondi, Visser, Ford, Schmidt, Birck, Georges, Mett, Walker, Boada, Drzezga, Vandenberghe, Hanseeuw, Jessen, Schöll, Ritchie, Lopes Alves, Gispert and Barkhof.
Conflict of interest statement
LC has received research support from GE Healthcare (paid to institution). GF is an employee of GE Healthcare. HP is an employee of GE Healthcare. DA received funding by the Fondation Recherche Alzheimer and the Swiss National Science Foundation (project CRSK-3_196354/1). PM received consulting fees from Oncovision. CB is an employee of GE Healthcare. GBF reports grants from Avid Radiopharmaceuticals, Biogen, GE International, Guerbert, IXICO, Merz Pharma, Nestlé, Novartis, Eisai, Piramal, Roche, Siemens, Teva Pharmaceutical Industries, and Vifor Pharma; he has received personal fees from AstraZeneca, Avid Radiopharmaceuticals, Biogen, Roche, Diadem, Neurodiem, Elan Pharmaceuticals, GE International, Lundbeck, Pfizer, and TauRx Therapeutics. AN received consulting fees from Hoffman La Roche. Patent: US patent alpha 7 nicotinic PET tracer. She is deputy chairman of Wennergren Foundations. PP has received research support from GE Healthcare (paid to institution). AS is an employee of Life Molecular Imaging GmbH. RG is an employee of Life Molecular Imaging GmbH. PV has served as member of the advisory board of Roche Diagnostics and received nonfinancial support from GE Healthcare, research support from Biogen and grants from Bristol-Myers Squibb, EU/EFPIA Innovative Medicines Initiative Joint Undertaking, EU Joint Programme–Neurodegenerative Disease Research (JPND and ZonMw). LF is an employee of Janssen Pharmaceuticals. Mark Schmidt is an employee of Janssen Pharmaceuticals. CB is an employee of Alzheimer Europe which has received grant support from Horizon2020, EU/EFPIA Innovative Medicines Initiative Joint Undertaking and the Luxembourg National Research Fund under the aegis of the EU Joint Programme - Neurodegenerative Disease Research (JPND) and sponsorship from AbbVie, Biogen, Danone, Eisai, GE Healthcare, Grifols, Janssen, Lilly, NovoNordisk, Roche and TauRx. JG is an employee of Alzheimer Europe which has received grant support from Horizon2020, EU/EFPIA Innovative Medicines Initiative Joint Undertaking and the Luxembourg National Research Fund under the aegis of the EU Joint Programme - Neurodegenerative Disease Research (JPND) and sponsorship from AbbVie, Biogen, Danone, Eisai, GE Healthcare, Grifols, Janssen, Lilly, NovoNordisk, Roche and TauRx. AM is an employee of GE Healthcare. MB has been a consultant for Araclon, Avid, Bayer, Elan, Grifols, Janssen/Pfizer, Lilly, Neuroptix, Nutricia, Roche, Sanofi, Biogen, and Servier; and received fees for lectures and funds for research from Araclon, Esteve, Grifols, Janssen, Novartis, Nutricia, Piramal, Pfizer-Wyett, Roche, and Servier. RV institution has Clinical Trial Agreements (RV as PI) with Alector, Biogen, Janssen Pharmaceuticals, NovoNordisk, Prevail, Roche, UCB. RV's institution has consultancy agreements (RV as DSMB member) with AC Immune and Novartis. RV was global PI of the Phase 1 and 2 18F-flutemetamol trials. RV's institution had a material transfer agreement (RV as PI) with GEHC for free tracer delivery of the FPACK cohort baseline scans and with Avid Pharmaceuticals, an EliLilly subsidiary. AD Research support: Siemens Healthineers, Life Molecular Imaging, GE Healthcare, AVID Radiopharmaceuticals, Sofie, Eisai, Novartis/AAA. Speaker Honorary/Advisory Boards: Siemens Healthineers, Sanofi, GE Healthcare, Biogen, Novo Nordisk, Invicro, Novartis/AAA, Bayer Vital. Stock: Siemens Healthineers, Lantheus Holding. Patents: Patent pending for 18F-PSMA7 (PSMA PET imaging tracer). BH received consulting fees from Biogen and Roche. The Belgian Foundation for Scientific Research supports his salary (FNRS CCL grant #40010417). He has received grants from the FNRS (including the WELBIO fund #0010035), the Belgian Alzheimer Research Foundation (SAO-FRA), the Louvain and Saint-Luc Foundations, and the Queen Elizabeth Medical Foundation (QEMF-FMRE). FJ received payment/honoraria from Roche and Lilly. He has participated on a Data Safety Monitoring Board or Advisory Board for AC Immune, Biogen, Roche, Eisai, and Grifols. MS has received fees for contributions to Advisory Boards from Biogen, Eisai, Eli Lilly, Roche, Roche Diagnostics, Actinogen, Alchemab, Merck, Kyowa Kirin and Signant. His research has been supported by Janssen and Biogen. He was Chief Investigator of the IMI Funded EPAD project that had multiple EFPIA and SME partners. CR has received fees for contributions to Advisory Boards from Biogen, Eisai, Eli Lilly, Roche, Roche Diagnostics, Actinogen, Alchemab, Merck, Kyowa Kirin, and Signant. His research has been supported by Janssen and Biogen. He was Chief Investigator of the IMI Funded EPAD project that had multiple EFPIA and SME partners. JG has received research support from GE Healthcare, Roche Diagnostics, F. Hoffmann – La Roche and speaker's fees from Biogen and Philips. In addition, he holds a “Ramón y Cajal” fellowship (RYC-2013-13054), has received research support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AMYPAD grant agreement no 115952, and from Ministerio de Ciencia y Universidades (grant agreement RTI2018-102261). FB Steering committee or iDMC member for Biogen, Merck, Roche, EISAI and Prothena. Consultant for Roche, Biogen, Merck, IXICO, Jansen, Combinostics. Research agreements with Merck, Biogen, GE Healthcare, Roche. Co-founder and shareholder of Queen Square Analytics LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Quantitative amyloid PET in Alzheimer's disease: the AMYPAD prognostic and natural history study.Alzheimers Dement. 2020 May;16(5):750-758. doi: 10.1002/alz.12069. Epub 2020 Apr 12. Alzheimers Dement. 2020. PMID: 32281303 Free PMC article. Clinical Trial.
-
Recruitment of pre-dementia participants: main enrollment barriers in a longitudinal amyloid-PET study.Alzheimers Res Ther. 2023 Nov 2;15(1):189. doi: 10.1186/s13195-023-01332-4. Alzheimers Res Ther. 2023. PMID: 37919783 Free PMC article.
-
Early detection of amyloid load using 18F-florbetaben PET.Alzheimers Res Ther. 2021 Mar 27;13(1):67. doi: 10.1186/s13195-021-00807-6. Alzheimers Res Ther. 2021. PMID: 33773598 Free PMC article.
-
Centiloid recommendations for clinical context-of-use from the AMYPAD consortium.Alzheimers Dement. 2024 Dec;20(12):9037-9048. doi: 10.1002/alz.14336. Epub 2024 Nov 20. Alzheimers Dement. 2024. PMID: 39564918 Free PMC article. Review.
-
Quantification of amyloid PET for future clinical use: a state-of-the-art review.Eur J Nucl Med Mol Imaging. 2022 Aug;49(10):3508-3528. doi: 10.1007/s00259-022-05784-y. Epub 2022 Apr 7. Eur J Nucl Med Mol Imaging. 2022. PMID: 35389071 Free PMC article. Review.
Cited by
-
The preservation of right cingulum fibers in subjective cognitive decline of preclinical phase of Alzheimer's disease.Front Aging Neurosci. 2023 Oct 30;15:1223697. doi: 10.3389/fnagi.2023.1223697. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37965494 Free PMC article.
-
Quantification Supports Amyloid PET Visual Assessment of Challenging Cases: Results from the AMYPAD Diagnostic and Patient Management Study.J Nucl Med. 2025 Jan 3;66(1):110-116. doi: 10.2967/jnumed.124.268119. J Nucl Med. 2025. PMID: 39542700 Free PMC article.
-
Amyloid-PET imaging predicts functional decline in clinically normal individuals.Alzheimers Res Ther. 2024 Jun 17;16(1):130. doi: 10.1186/s13195-024-01494-9. Alzheimers Res Ther. 2024. PMID: 38886831 Free PMC article.
-
From Innovator Result-driven to Multi-actor Impact-oriented Public-Private Partnerships: Integrating the Patient Perspective.Handb Exp Pharmacol. 2024;286:137-168. doi: 10.1007/164_2024_730. Handb Exp Pharmacol. 2024. PMID: 39235487 Review.
-
Revisiting Centiloids using AI.Res Sq [Preprint]. 2025 Jul 8:rs.3.rs-7015694. doi: 10.21203/rs.3.rs-7015694/v1. Res Sq. 2025. PMID: 40671806 Free PMC article. Preprint.
References
-
- Barthel H, Butzke D, Diemling M, Senda M, Sattler B, Seibyl J, et al. . Florbetaben PET and the Hermes BRASS tool for automated regional and voxelwise quantification of β-amyloid brain load. Soc Nuclear Med. (2011). 10.1016/j.jalz.2011.05.078 - DOI
-
- Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. . Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the Alzheimer's association. J Nucl Med. (2013) 54:476–90. 10.2967/jnumed.113.120618 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

