Therapeutic potential of blocking GAPDH nitrosylation with CGP3466b in experimental autoimmune encephalomyelitis
- PMID: 36761918
- PMCID: PMC9902867
- DOI: 10.3389/fneur.2022.979659
Therapeutic potential of blocking GAPDH nitrosylation with CGP3466b in experimental autoimmune encephalomyelitis
Abstract
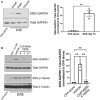
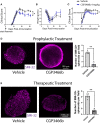
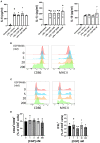
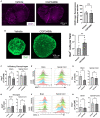
Multiple sclerosis (MS) is a neuroinflammatory disease of the central nervous system (CNS). Although classically considered a demyelinating disease, neuroaxonal injury occurs in both the acute and chronic phases and represents a pathologic substrate of disability not targeted by current therapies. Nitric oxide (NO) generated by CNS macrophages and microglia contributes to neuroaxonal injury in all phases of MS, but candidate therapies that prevent NO-mediated injury have not been identified. Here, we demonstrate that the multifunctional protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is robustly nitrosylated in the CNS in the experimental autoimmune encephalomyelitis (EAE) mouse model of MS. GAPDH nitrosylation is blocked in vivo with daily administration of CGP3466b, a CNS-penetrant compound with an established safety profile in humans. Consistent with the known role of nitrosylated GAPDH (SNO-GAPDH) in neuronal cell death, blockade of SNO-GAPDH with CGP3466b attenuates neurologic disability and reduces axonal injury in EAE independent of effects on the immune system. Our findings suggest that SNO-GAPDH contributes to neuroaxonal injury during neuroinflammation and identify CGP3466b as a candidate neuroprotective therapy in MS.
Keywords: GAPDH; experimental autoimmune encephalomyelitis; multiple sclerosis; neuroinflammation; neuroprotection; nitric oxide; nitrosylation.
Copyright © 2023 Godfrey, Hwang, Cho, Shanmukha, Gharibani, Abramson and Kornberg.
Conflict of interest statement
MK has received consulting fees from Biogen Idec, Genentech, Janssen Pharmaceuticals, Novartis, OptumRx, and TG Therapeutics on topics unrelated to this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Nitrosylation of GAPDH augments pathological tau acetylation upon exposure to amyloid-β.Sci Signal. 2018 Mar 20;11(522):eaao6765. doi: 10.1126/scisignal.aao6765. Sci Signal. 2018. PMID: 29559585 Free PMC article.
-
Products of S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase: Relation between S-nitrosylation and oxidation.Biochim Biophys Acta Gen Subj. 2022 Jan;1866(1):130032. doi: 10.1016/j.bbagen.2021.130032. Epub 2021 Oct 7. Biochim Biophys Acta Gen Subj. 2022. PMID: 34627945
-
Connexin 30 Deficiency Attenuates Chronic but Not Acute Phases of Experimental Autoimmune Encephalomyelitis Through Induction of Neuroprotective Microglia.Front Immunol. 2018 Nov 7;9:2588. doi: 10.3389/fimmu.2018.02588. eCollection 2018. Front Immunol. 2018. PMID: 30464764 Free PMC article.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
-
The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis.Acta Neuropathol. 2019 May;137(5):757-783. doi: 10.1007/s00401-019-01980-7. Epub 2019 Mar 7. Acta Neuropathol. 2019. PMID: 30847559 Free PMC article. Review.
Cited by
-
Nitric Oxide Signaling and Sensing in Age-Related Diseases.Antioxidants (Basel). 2024 Oct 9;13(10):1213. doi: 10.3390/antiox13101213. Antioxidants (Basel). 2024. PMID: 39456466 Free PMC article. Review.
-
Genome-Wide Screening in Human Embryonic Stem Cells Highlights the Hippo Signaling Pathway as Granting Synthetic Viability in ATM Deficiency.Cells. 2023 May 29;12(11):1503. doi: 10.3390/cells12111503. Cells. 2023. PMID: 37296624 Free PMC article.
-
TPPB modulates PKC activity to attenuate neuroinflammation and ameliorate experimental multiple sclerosis.Front Cell Neurosci. 2024 May 22;18:1373557. doi: 10.3389/fncel.2024.1373557. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38841204 Free PMC article.
-
Phosphoglycerate mutase regulates Treg differentiation through control of serine synthesis and one-carbon metabolism.Elife. 2025 Jul 28;14:RP104423. doi: 10.7554/eLife.104423. Elife. 2025. PMID: 40720256 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

