Predictors of residual disease after debulking surgery in advanced stage ovarian cancer
- PMID: 36761962
- PMCID: PMC9902593
- DOI: 10.3389/fonc.2023.1090092
Predictors of residual disease after debulking surgery in advanced stage ovarian cancer
Abstract
Objective: Optimal debulking with no macroscopic residual disease strongly predicts ovarian cancer survival. The ability to predict likelihood of optimal debulking, which may be partially dependent on tumor biology, could inform clinical decision-making regarding use of neoadjuvant chemotherapy. Thus, we developed a prediction model including epidemiological factors and tumor markers of residual disease after primary debulking surgery.
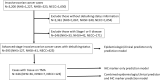
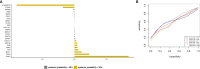
Methods: Univariate analyses examined associations of 11 pre-diagnosis epidemiologic factors (n=593) and 24 tumor markers (n=204) with debulking status among incident, high-stage, epithelial ovarian cancer cases from the Nurses' Health Studies and New England Case Control study. We used Bayesian model averaging (BMA) to develop prediction models of optimal debulking with 5x5-fold cross-validation and calculated the area under the curve (AUC).
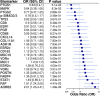
Results: Current aspirin use was associated with lower odds of optimal debulking compared to never use (OR=0.52, 95%CI=0.31-0.86) and two tissue markers, ADRB2 (OR=2.21, 95%CI=1.23-4.41) and FAP (OR=1.91, 95%CI=1.24-3.05) were associated with increased odds of optimal debulking. The BMA selected aspirin, parity, and menopausal status as the epidemiologic/clinical predictors with the posterior effect probability ≥20%. While the prediction model with epidemiologic/clinical predictors had low performance (average AUC=0.49), the model adding tissue biomarkers showed improved, but weak, performance (average AUC=0.62).
Conclusions: Addition of ovarian tumor tissue markers to our multivariable prediction models based on epidemiologic/clinical data slightly improved the model performance, suggesting debulking status may be in part driven by tumor characteristics. Larger studies are warranted to identify those at high risk of poor surgical outcomes informing personalized treatment.
Keywords: debulking; immunohistochemistry; ovarian cancer; prediction model; residual disease; tissue microarray.
Copyright © 2023 Abbas-Aghababazadeh, Sasamoto, Townsend, Huang, Terry, Vitonis, Elias, Poole, Hecht, Tworoger and Fridley.
Conflict of interest statement
NS reports grants from NCI, DOD, Marsha Rivkin Center for Ovarian Cancer Research. TH report grants from NHLBI. KLT reports grants from NIH. KE reports funding from Abcam; royalties from Aspira Women’s Health; consultant of Bluestar Genomics; personal fees from Expert Institute; and is a member of the Enhanced Recovery After Surgery USA unpaid. EP reports grants from NIH. SST reports grants from NIH/NCI, DOD, Rivkin Center, State of Florida, BMS; personal fees from AACR, Ponce Health Science University, Ovarian Cancer Research Alliance, German Cancer Research Center, Harvard T.H. Chan School of Public Health, and NIH outside of submitted work; and is a member of external advisory committee of California Teachers Study City of Hope and The Tomorrow Project Alberta Cancer Center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Primary debulking surgery versus primary neoadjuvant chemotherapy for high grade advanced stage ovarian cancer: comparison of survivals.Radiol Oncol. 2018 Sep 11;52(3):307-319. doi: 10.2478/raon-2018-0030. Radiol Oncol. 2018. PMID: 30210049 Free PMC article.
-
Laparoscopy for diagnosing resectability of disease in women with advanced ovarian cancer.Cochrane Database Syst Rev. 2019 Mar 23;3(3):CD009786. doi: 10.1002/14651858.CD009786.pub3. Cochrane Database Syst Rev. 2019. PMID: 30907434 Free PMC article.
-
Primary debulking surgery vs. neoadjuvant chemotherapy followed by interval debulking surgery for patients with advanced ovarian cancer.Arch Gynecol Obstet. 2016 Jan;293(1):163-168. doi: 10.1007/s00404-015-3813-z. Epub 2015 Jul 22. Arch Gynecol Obstet. 2016. PMID: 26198168
-
A novel classification of residual disease after interval debulking surgery for advanced-stage ovarian cancer to better distinguish oncologic outcome.Am J Obstet Gynecol. 2019 Oct;221(4):326.e1-326.e7. doi: 10.1016/j.ajog.2019.05.006. Epub 2019 May 10. Am J Obstet Gynecol. 2019. PMID: 31082382
-
Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian/fallopian tube/primary peritoneal cancer.Cochrane Database Syst Rev. 2018 Oct 8;10(10):CD012567. doi: 10.1002/14651858.CD012567.pub2. Cochrane Database Syst Rev. 2018. PMID: 30298516 Free PMC article.
Cited by
-
Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment.Int J Mol Sci. 2024 Mar 2;25(5):2925. doi: 10.3390/ijms25052925. Int J Mol Sci. 2024. PMID: 38474178 Free PMC article. Review.
References
-
- American Cancer Society . Cancer facts & figures 2021. Atlanta: American Cancer Society, American Cancer Society; (2021).
-
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the arbeitsgemeinschaft gynaekologische onkologie studiengruppe ovarialkarzinom (AGO-OVAR) and the groupe d’Investigateurs nationaux pour les etudes des cancers de l’Ovaire (GINECO). Cancer (2009) 115(6):1234–44. doi: 10.1002/cncr.24149 - DOI - PubMed
-
- Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. . Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of gynecologic oncology and American society of clinical oncology clinical practice guideline. J Clin Oncol (2016) 34(28):3460–73. doi: 10.1200/jco.2016.68.6907 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

