A fast and accurate mouse model for inducing non-alcoholic steatohepatitis
- PMID: 36762217
- PMCID: PMC9876774
- DOI: 10.22037/ghfbb.v15i4.2593
A fast and accurate mouse model for inducing non-alcoholic steatohepatitis
Abstract
Aim: This study aimed to perform a head-to-head comparison of changes during NASH progression throughout 6-11 weeks of an experiment to supply a faster nutritional model in mimicking NASH to decrease the duration and cost of in vivo studies.
Background: New therapies are urgently needed because of the growing prevalence of non-alcoholic steatohepatitis (NASH) and the lack of an effective treatment approach. Currently, dietary interventions are the most efficient options.
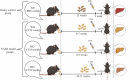
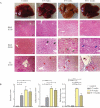
Methods: This study compared features of NASH in a murine model using protocol that combined special nutritional regimes based on the combination of 21.1% fat, 41% sucrose, and 1.25% cholesterol with weekly intraperitoneal injections of carbon tetrachloride (CCl4). Male C57BL/6J mice received either special compositions + CCl4 (NASH group) or standard chow diet (healthy control group) for 11 weeks. Liver histopathology based on hematoxylin and eosin (H&E) and Masson's Trichrome (TC) staining and biochemical analyses were used to assess disease progression.
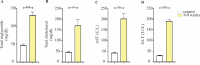
Results: In C57BL/6J mice administered a high fat, high cholesterol, high sucrose diet and CCl4 for 8 weeks, steatohepatitis with pronounced hepatocyte ballooning, inflammation, steatosis, and fibrosis was observed. According to the NAFLD activity scoring system, the maximum NAS score was manifested after 8-9 weeks (NAS score: 6.75). Following this protocol also led to a significant increase in AST and ALT, total cholesterol, and total triglyceride serum levels in the NASH group.
Conclusion: Following the special nutritional regime based on high fat, cholesterol, and sucrose in combination with CCL4 injections resulted in a NASH model using C57BL/6J mice in a shorter time compared to similar studies. The obtained histopathological NASH features can be advantageous for preclinical drug testing.
Keywords: Animal model; Carbon tetrachloride; Liver diseases; Nonalcoholic; Steatohepatitis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Carbon tetrachloride (CCl4) accelerated development of non-alcoholic fatty liver disease (NAFLD)/steatohepatitis (NASH) in MS-NASH mice fed western diet supplemented with fructose (WDF).BMC Gastroenterol. 2020 Oct 15;20(1):339. doi: 10.1186/s12876-020-01467-w. BMC Gastroenterol. 2020. PMID: 33059584 Free PMC article.
-
Obese diet-induced mouse models of nonalcoholic steatohepatitis-tracking disease by liver biopsy.World J Hepatol. 2016 Jun 8;8(16):673-84. doi: 10.4254/wjh.v8.i16.673. World J Hepatol. 2016. PMID: 27326314 Free PMC article.
-
Novel non-alcoholic steatohepatitis model with histopathological and insulin-resistant features.Pathol Int. 2018 Jan;68(1):12-22. doi: 10.1111/pin.12612. Epub 2017 Nov 20. Pathol Int. 2018. PMID: 29154469
-
Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis.Hepatology. 2019 May;69(5):2241-2257. doi: 10.1002/hep.30333. Hepatology. 2019. PMID: 30372785 Review.
-
Non-alcoholic steatohepatitis in children.Pediatr Transplant. 2004 Dec;8(6):613-8. doi: 10.1111/j.1399-3046.2004.00241.x. Pediatr Transplant. 2004. PMID: 15598336 Review.
Cited by
-
Notch1 siRNA and AMD3100 Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease.Biomedicines. 2025 Feb 16;13(2):486. doi: 10.3390/biomedicines13020486. Biomedicines. 2025. PMID: 40002899 Free PMC article.
-
Exploring the antioxidant and protective effects of Marsdenia thyrsiflora Hook.f. leaf extract against carbon tetrachloride-induced hepatic damage in rat models.Front Pharmacol. 2024 Oct 22;15:1463922. doi: 10.3389/fphar.2024.1463922. eCollection 2024. Front Pharmacol. 2024. PMID: 39502533 Free PMC article.
References
-
- Dufour JF, Scherer R, Balp MM, McKenna SJ, Janssens N, Lopez P, et al. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–A targeted literature review. Endocr Metab Sci. 2021;3:100089.
-
- Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. Jama. 2020;323:1175–1183. - PubMed
-
- Thrasher T, Abdelmalek MF. Nonalcoholic fatty liver disease. N C Med J. 2016;77:216–219. - PubMed
LinkOut - more resources
Full Text Sources
