Implications of S-glutathionylation of sarcomere proteins in cardiac disorders, therapies, and diagnosis
- PMID: 36762302
- PMCID: PMC9902711
- DOI: 10.3389/fcvm.2022.1060716
Implications of S-glutathionylation of sarcomere proteins in cardiac disorders, therapies, and diagnosis
Abstract
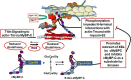
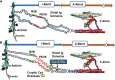
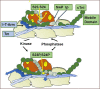
The discovery that cardiac sarcomere proteins are substrates for S-glutathionylation and that this post-translational modification correlates strongly with diastolic dysfunction led to new concepts regarding how levels of oxidative stress affect the heartbeat. Major sarcomere proteins for which there is evidence of S-glutathionylation include cardiac myosin binding protein C (cMyBP-C), actin, cardiac troponin I (cTnI) and titin. Our hypothesis is that these S-glutathionylated proteins are significant factors in acquired and familial disorders of the heart; and, when released into the serum, provide novel biomarkers. We consider the molecular mechanisms for these effects in the context of recent revelations of how these proteins control cardiac dynamics in close collaboration with Ca2+ fluxes. These revelations were made using powerful approaches and technologies that were focused on thin filaments, thick filaments, and titin filaments. Here we integrate their regulatory processes in the sarcomere as modulated mainly by neuro-humoral control of phosphorylation inasmuch evidence indicates that S-glutathionylation and protein phosphorylation, promoting increased dynamics and modifying the Frank-Starling relation, may be mutually exclusive. Earlier studies demonstrated that in addition to cTnI as a well-established biomarker for cardiac disorders, serum levels of cMyBP-C are also a biomarker for cardiac disorders. We describe recent studies approaching the question of whether serum levels of S-glutathionylated-cMyBP-C could be employed as an important clinical tool in patient stratification, early diagnosis in at risk patients before HFpEF, determination of progression, effectiveness of therapeutic approaches, and as a guide in developing future therapies.
Keywords: cardiac troponin I; cross-bridge kinetics; length dependent activation; myosin binding protein C; protein phosphorylation; titin.
Copyright © 2023 Rosas and Solaro.
Conflict of interest statement
RS was a member of the Scientific Advisor Board of Cytokinetics, Inc. and a consultant to MyoKardia/Bristol-Myers-Squibb, and Edgewise Pharmaceuticals. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

