HIV-1 transmission: modelling and direct visualization in the third dimension
- PMID: 36762762
- PMCID: PMC10332454
- DOI: 10.1093/jmicro/dfad014
HIV-1 transmission: modelling and direct visualization in the third dimension
Abstract
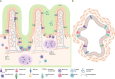
Identifying initial events of mucosal entry of human immunodeficiency virus type-1 (HIV-1) in laboratory-based, physiologically relevant and high-throughput contexts may aid in designing effective strategies to block local transmission and spread of HIV-1. Several paradigms have been posited for how HIV-1 crosses mucosal barriers to establish infection based on two dimensional (2D) culture-based or animal-based models. Nevertheless, despite these models stemming from 2D culture and animal studies, monolayers of cells poorly replicate the complex niche that influences viral entry at mucosal surfaces, whereas animal models often inadequately reproduce human disease pathophysiology and are prohibitively expensive. Organoids, having never been directly utilized in HIV-1 transmission investigations, may offer a compromise between 2D culture and animal models as they provide a platform that mimics the biophysical and biochemical niche of mucosal tissues. Importantly, observation of events downstream of viral inoculation is potentially accessible to researchers via an array of microscopy techniques. Because of the potential insights organoids may provide in this context, we offer this review to highlight key physiological factors of HIV-1 transmission at common mucosal sites and a discussion to highlight how many of these factors can be recapitulated in organoids, their current limitations and what questions can initially be addressed, particularly using a selective inclusion of quantitative light microscopy techniques. Harnessing organoids for direct observation of HIV-1 entry at mucosal sites may uncover potential therapeutic targets which prevent the establishment of HIV-1 infection.
Keywords: HIV-1 entry; imaging HIV-1 in tissue; mucosal immunity; two-photon FLIM metabolism; two-photon microscopy.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Japanese Society of Microscopy. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to That of Nonneutralizing Antibodies.J Virol. 2016 Dec 16;91(1):e01762-16. doi: 10.1128/JVI.01762-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795431 Free PMC article.
-
Modeling mucosal cell-associated HIV type 1 transmission in vitro.J Infect Dis. 2014 Dec 15;210 Suppl 3(Suppl 3):S648-53. doi: 10.1093/infdis/jiu537. J Infect Dis. 2014. PMID: 25414419 Free PMC article. Review.
-
Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1.Immunity. 2007 Feb;26(2):257-70. doi: 10.1016/j.immuni.2007.01.007. Immunity. 2007. PMID: 17306567 Free PMC article.
-
[Application of human mucosal explants culture in the HIV-1 sexual transmission].Sheng Wu Gong Cheng Xue Bao. 2011 Feb;27(2):172-9. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 21650041 Review. Chinese.
-
CD169-dependent cell-associated HIV-1 transmission: a driver of virus dissemination.J Infect Dis. 2014 Dec 15;210 Suppl 3(Suppl 3):S641-7. doi: 10.1093/infdis/jiu442. J Infect Dis. 2014. PMID: 25414418 Free PMC article. Review.
References
-
- UNAIDS (2016) Global AIDS Update 2016. World Health Organization, 422.
-
- Shattock R J et al. (2011) AIDS. Turning the tide against HIV. Science (1979) 333: 42–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

