Nucleolar stress: Molecular mechanisms and related human diseases
- PMID: 36762786
- PMCID: PMC10154868
- DOI: 10.1111/cas.15755
Nucleolar stress: Molecular mechanisms and related human diseases
Abstract
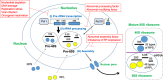
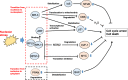
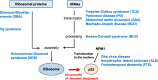
Ribosome biogenesis in the nucleolus is an important process that consumes 80% of a cell's intracellular energy supply. Disruption of this process results in nucleolar stress, triggering the activation of molecular systems that respond to this stress to maintain homeostasis. Although nucleolar stress was originally thought to be caused solely by abnormalities of ribosomal RNA (rRNA) and ribosomal proteins (RPs), an accumulating body of more current evidence suggests that many other factors, including the DNA damage response and oncogenic stress, are also involved in nucleolar stress response signaling. Cells reacting to nucleolar stress undergo cell cycle arrest or programmed death, mainly driven by activation of the tumor suppressor p53. This observation has nominated nucleolar stress as a promising target for cancer therapy. However, paradoxically, some RP mutations have also been implicated in cancer initiation and progression, necessitating caution. In this article, we summarize recent findings on the molecular mechanisms of nucleolar stress and the human ribosomal diseases and cancers that arise in its wake.
Keywords: PICT1; RPL11; nucleolar stress; p53; ribosomal disease; signal transduction.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
A.S. is a current member of the Editorial Board of
Figures


Similar articles
-
The roles of RRP15 in nucleolar formation, ribosome biogenesis and checkpoint control in human cells.Oncotarget. 2017 Feb 21;8(8):13240-13252. doi: 10.18632/oncotarget.14658. Oncotarget. 2017. PMID: 28099941 Free PMC article.
-
A new PICTure of nucleolar stress.Cancer Sci. 2012 Apr;103(4):632-7. doi: 10.1111/j.1349-7006.2012.02219.x. Epub 2012 Mar 8. Cancer Sci. 2012. PMID: 22320853 Free PMC article. Review.
-
Perturbation of RNA Polymerase I transcription machinery by ablation of HEATR1 triggers the RPL5/RPL11-MDM2-p53 ribosome biogenesis stress checkpoint pathway in human cells.Cell Cycle. 2018;17(1):92-101. doi: 10.1080/15384101.2017.1403685. Epub 2017 Dec 10. Cell Cycle. 2018. PMID: 29143558 Free PMC article.
-
Nucleolar stress: From development to cancer.Semin Cell Dev Biol. 2023 Feb 28;136:64-74. doi: 10.1016/j.semcdb.2022.04.001. Epub 2022 Apr 8. Semin Cell Dev Biol. 2023. PMID: 35410715 Free PMC article. Review.
-
Acrolein preferentially damages nucleolus eliciting ribosomal stress and apoptosis in human cancer cells.Oncotarget. 2016 Dec 6;7(49):80450-80464. doi: 10.18632/oncotarget.12608. Oncotarget. 2016. PMID: 27741518 Free PMC article.
Cited by
-
Contribution of p53-dependent and -independent mechanisms to upregulation of p21 in Fanconi anemia.PLoS Genet. 2024 Nov 7;20(11):e1011474. doi: 10.1371/journal.pgen.1011474. eCollection 2024 Nov. PLoS Genet. 2024. PMID: 39509458 Free PMC article.
-
Impacts of ribosomal RNA sequence variation on gene expression and phenotype.Philos Trans R Soc Lond B Biol Sci. 2025 Mar 6;380(1921):20230379. doi: 10.1098/rstb.2023.0379. Epub 2025 Mar 6. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40045785 Free PMC article. Review.
-
Essential role of Dhx16-mediated ribosome assembly in maintenance of hematopoietic stem cells.Leukemia. 2024 Dec;38(12):2699-2708. doi: 10.1038/s41375-024-02423-3. Epub 2024 Sep 27. Leukemia. 2024. PMID: 39333759
-
Bypassing the guardian: regulated cell death pathways in p53-mutant cancers.Cell Mol Biol Lett. 2025 Jun 14;30(1):68. doi: 10.1186/s11658-025-00751-5. Cell Mol Biol Lett. 2025. PMID: 40517236 Free PMC article. Review.
-
Ribosome Biogenesis and Function in Cancer: From Mechanisms to Therapy.Cancers (Basel). 2025 Jul 31;17(15):2534. doi: 10.3390/cancers17152534. Cancers (Basel). 2025. PMID: 40805230 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

