Preexisting evidence and outcome of phase III trials in gastrointestinal oncology: a systematic review
- PMID: 36762842
- PMCID: PMC10165488
- DOI: 10.1093/jnci/djad030
Preexisting evidence and outcome of phase III trials in gastrointestinal oncology: a systematic review
Abstract
Background: A minority of phase III trials in gastrointestinal oncology are positive. We assessed the association between their outcome and the level and characteristics of preexisting evidence.
Methods: EMBASE, PubMed, and proceedings from international meetings were searched for phase III gastrointestinal cancer trials (gastroesophageal, hepatocellular, biliary tract, pancreatic, small bowel, colorectal, anal, stromal, and neuroendocrine) between January 2000 and June 2020. Trials investigating anticancer drugs for advanced disease, with superiority design and standard treatments as control were eligible. The highest level of preexisting evidence was retrieved from the main study report.
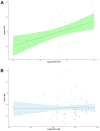
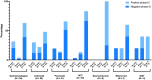
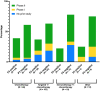
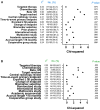
Results: A total of 193 phase III trials were included, and 69 (35.8%) met their primary endpoint. Positivity rates were as follows: gastroesophageal 37%, colorectal 48%, pancreatic 17.1%, hepatocellular 20%, neuroendocrine 75%, and both biliary tract and GIST 60%. No information about preexisting evidence was found for 44 trials (22.8%). For the remaining 149, preexisting evidence consisted of phase II studies in 123 cases (82.6%) and phase I studies in 26 cases (17.4%). The probability of success was 34.1%, 35.8%, and 35.7%, respectively (P = .934). No parameter from prior studies predicted the outcome of phase III trials except β < .2 (P = .048). A numerically increased success rate was observed for phase III trials preceded by positive phase II studies (41.9% vs 18.5%, P = .2).
Conclusions: There does not appear to be an association between level of prior evidence and success of phase III gastrointestinal cancer trials. These data, along with the high phase III failure rate, highlight the need to improve the drug development process in this setting.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
G.B.—Travel grants: Amgen. A.H.—Consultancy, advisory roles, honoraria: Amgen, Bayer, Eli Lilly, Merck, Pierre Fabre, Servier, Sirtex. Research funding (institutional): Amgen, Astra Zeneca, Ipsen, Leo Pharma, Merck, Roche, Sanofi, Teva Pharma. Travel grants: Merck, Roche, Sirtex. M.D.M.—Consultancy, advisory roles, honoraria: Astra Zeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Takeda; Research funding (institutional): Beigene, Exelixis, Merck Sharp & Dohme, Pfizer, Roche, Tesaro—GlaxoSmithKline. FS—Consultancy, advisory roles, honoraria: AMAL Therapeutics, Bayer, BMS, Dragonfly Therapeutics, Merck, Nordic Pharma, Roche, Servier; Research funding (institutional): Amgen, Astra Zeneca, Bayer, BMS, Roche, Sanofi; Travel grants: Amgen, Bayer, Lilly, Servier; Leadership roles: Co-Chair EORTC Task Force Colon, Rectum, Anal Canal. All other authors do not have any conflicts of interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

