Phytopathogenic bacteria utilize host glucose as a signal to stimulate virulence through LuxR homologues
- PMID: 36762904
- PMCID: PMC10013830
- DOI: 10.1111/mpp.13302
Phytopathogenic bacteria utilize host glucose as a signal to stimulate virulence through LuxR homologues
Abstract
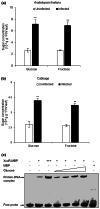
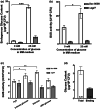
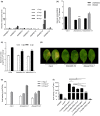
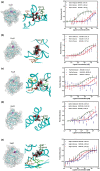
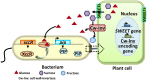
Chemical signal-mediated biological communication is common within bacteria and between bacteria and their hosts. Many plant-associated bacteria respond to unknown plant compounds to regulate bacterial gene expression. However, the nature of the plant compounds that mediate such interkingdom communication and the underlying mechanisms remain poorly characterized. Xanthomonas campestris pv. campestris (Xcc) causes black rot disease on brassica vegetables. Xcc contains an orphan LuxR regulator (XccR) which senses a plant signal that was validated to be glucose by HPLC-MS. The glucose concentration increases in apoplast fluid after Xcc infection, which is caused by the enhanced activity of plant sugar transporters translocating sugar and cell-wall invertases releasing glucose from sucrose. XccR recruits glucose, but not fructose, sucrose, glucose 6-phosphate, and UDP-glucose, to activate pip expression. Deletion of the bacterial glucose transporter gene sglT impaired pathogen virulence and pip expression. Structural prediction showed that the N-terminal domain of XccR forms an alternative pocket neighbouring the AHL-binding pocket for glucose docking. Substitution of three residues affecting structural stability abolished the ability of XccR to bind to the luxXc box in the pip promoter. Several other XccR homologues from plant-associated bacteria can also form stable complexes with glucose, indicating that glucose may function as a common signal molecule for pathogen-plant interactions. The conservation of a glucose/XccR/pip-like system in plant-associated bacteria suggests that some phytopathogens have evolved the ability to utilize host compounds as virulence signals, indicating that LuxRs mediate an interkingdom signalling circuit.
Keywords: LuxR ligand; glucose; interkingdom signalling; quorum sensing.
© 2023 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures

Similar articles
-
A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris.Mol Microbiol. 2007 Jul;65(1):121-36. doi: 10.1111/j.1365-2958.2007.05775.x. Mol Microbiol. 2007. PMID: 17581124
-
XerR, a negative regulator of XccR in Xanthomonas campestris pv. campestris, relieves its repressor function in planta.Cell Res. 2011 Jul;21(7):1131-42. doi: 10.1038/cr.2011.64. Epub 2011 Apr 12. Cell Res. 2011. PMID: 21483448 Free PMC article.
-
Synergistic activation of the pathogenicity-related proline iminopeptidase gene in Xanthomonas campestris pv. campestris by HrpX and a LuxR homolog.Appl Environ Microbiol. 2012 Oct;78(19):7069-74. doi: 10.1128/AEM.01726-12. Epub 2012 Aug 3. Appl Environ Microbiol. 2012. PMID: 22865058 Free PMC article.
-
The roles of histidine kinases in sensing host plant and cell-cell communication signal in a phytopathogenic bacterium.Philos Trans R Soc Lond B Biol Sci. 2019 Mar 4;374(1767):20180311. doi: 10.1098/rstb.2018.0311. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30967026 Free PMC article. Review.
-
Chemical signaling between plants and plant-pathogenic bacteria.Annu Rev Phytopathol. 2013;51:17-37. doi: 10.1146/annurev-phyto-082712-102239. Annu Rev Phytopathol. 2013. PMID: 23915131 Review.
Cited by
-
Constructing a Novel Disease Resistance Mechanism Model for Cruciferous Crops: An Example From Black Rot.Mol Plant Pathol. 2025 Feb;26(2):e70060. doi: 10.1111/mpp.70060. Mol Plant Pathol. 2025. PMID: 39924905 Free PMC article. Review.
-
Genome-wide identification and biochemical characterization of glycoside hydrolase gene family members in Tilletia Horrida.Mol Biol Rep. 2024 Nov 9;51(1):1136. doi: 10.1007/s11033-024-10059-w. Mol Biol Rep. 2024. PMID: 39520598
-
Starving the enemy: how plant and microbe compete for sugar on the border.Front Plant Sci. 2023 Aug 2;14:1230254. doi: 10.3389/fpls.2023.1230254. eCollection 2023. Front Plant Sci. 2023. PMID: 37600180 Free PMC article. Review.
-
The link between changing in host carbon allocation and resistance to Magnaporthe oryzae: a possible tactic for mitigating the rice blast fungus.Plant Signal Behav. 2024 Dec 31;19(1):2326870. doi: 10.1080/15592324.2024.2326870. Epub 2024 Mar 11. Plant Signal Behav. 2024. PMID: 38465846 Free PMC article. Review.
-
The role of sugar transporters in the battle for carbon between plants and pathogens.Plant Biotechnol J. 2024 Oct;22(10):2844-2858. doi: 10.1111/pbi.14408. Epub 2024 Jun 16. Plant Biotechnol J. 2024. PMID: 38879813 Free PMC article. Review.
References
-
- Ahmer, B.M. (2004) Cell‐to‐cell signalling in Escherichia coli and Salmonella enterica . Molecular Microbiology, 52, 933–945. - PubMed
-
- Berger, S. , Sinha, A.K. & Roitsch, T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. Journal of Experimental Botany, 58, 4019–4026. - PubMed
-
- Bonfig, K.B. , Gabler, A. , Simon, U.K. , Luschin‐Ebengreuth, N. , Hatz, M. , Berger, S. et al. (2010) Post‐translational derepression of invertase activity in source leaves via down‐regulation of invertase inhibitor expression is part of the plant defense response. Molecular Plant, 3, 1037–1048. - PubMed
-
- Bottomley, M.J. , Muraglia, E. , Bazzo, R. & Carfi, A. (2007) Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. Journal of Biological Chemistry, 282, 13592–13600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

