Effective Inhibition of Thyroid Antigen Presentation Using Retro-Inverso Peptides in Experimental Autoimmune Thyroiditis: A Pathway Toward Immune Therapies of Thyroid Autoimmunity
- PMID: 36762945
- PMCID: PMC10325802
- DOI: 10.1089/thy.2022.0511
Effective Inhibition of Thyroid Antigen Presentation Using Retro-Inverso Peptides in Experimental Autoimmune Thyroiditis: A Pathway Toward Immune Therapies of Thyroid Autoimmunity
Abstract
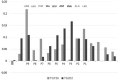
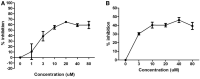
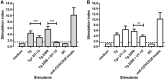
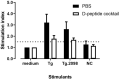
Background: Autoimmune thyroid diseases (AITD) represent the most common autoimmune diseases. However, current therapies focus on relieving the symptoms instead of curing AITD, and new therapies to reverse the autoimmune attack on the thyroid are needed. HLA-DRβ1-Arg74 is the key HLA class II allele that triggers AITD by presenting pathogenic thyroglobulin (Tg) peptides that activate thyroid self-reactive T cells. We hypothesized that blocking the presentation of Tg peptides to T cells within the HLA-DRβ1-Arg74 peptide binding cleft could reverse the autoimmune response to the thyroid in AITD. Methods: The approach we used to block Tg peptide presentation within HLA-DRβ1-Arg74 is to design retro-inverso D-amino acid (RID) peptides that have high affinity to the HLA-DRβ1-Arg74 peptide binding pocket. Results: By using computational approaches and molecular dynamics simulations, we designed two RID peptides, RT-15 and VT-15, that blocked peptide binding to recombinant HLA-DRβ1-Arg74 molecule, as well as T cell activation in vitro. Furthermore, RT-15 and VT-15 blocked in vivo T cell activation by thyroglobulin in humanized NOD-DR3 mice induced with experimental autoimmune thyroiditis. Conclusions: In summary, we discovered two RID peptides that block thyroglobulin peptide binding to HLA-DRβ1-Arg74 and their presentation to T cells in AITD. These findings set the stage for a personalized medicine therapeutic approach for AITD patients who carry the DRβ1-Arg74 allele. This antigen-specific therapeutic strategy can potentially be extended to other autoimmune diseases.
Keywords: Graves'disease; HLA-DR3; Hashimoto's thyroiditis; autoimmune thyroiditis; peptides.
Conflict of interest statement
Dr. Tomer declares that he was previously (2015–2017) the principal investigator on a basic research project jointly funded by the Juvenile Diabetes Research Foundation and Pfizer. The current article is not related to that research project. Drs. Li, Osman, and Tomer declare that they submitted a patent application that is not related to the content of this article. All other authors have no potential conflict of interest to declare.
Figures

Similar articles
-
Tg.2098 is a major human thyroglobulin T-cell epitope.J Autoimmun. 2010 Aug;35(1):45-51. doi: 10.1016/j.jaut.2010.01.004. Epub 2010 Mar 19. J Autoimmun. 2010. PMID: 20303712 Free PMC article.
-
Identifying a Small Molecule Blocking Antigen Presentation in Autoimmune Thyroiditis.J Biol Chem. 2016 Feb 19;291(8):4079-90. doi: 10.1074/jbc.M115.694687. Epub 2015 Dec 24. J Biol Chem. 2016. PMID: 26703475 Free PMC article.
-
Cepharanthine blocks TSH receptor peptide presentation by HLA-DR3: Therapeutic implications to Graves' disease.J Autoimmun. 2020 Mar;108:102402. doi: 10.1016/j.jaut.2020.102402. Epub 2020 Jan 21. J Autoimmun. 2020. PMID: 31980336 Free PMC article.
-
[The role of hereditary and environmental factors in autoimmune thyroid diseases].Orv Hetil. 2012 Jul 1;153(26):1013-22. doi: 10.1556/OH.2012.29370. Orv Hetil. 2012. PMID: 22735372 Review. Hungarian.
-
The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology.J Autoimmun. 2008 Feb-Mar;30(1-2):58-62. doi: 10.1016/j.jaut.2007.11.010. Epub 2008 Jan 4. J Autoimmun. 2008. PMID: 18178059 Free PMC article. Review.
Cited by
-
Associations between immune cell traits and autoimmune thyroid diseases: a bidirectional two-sample mendelian randomization study.Immunogenetics. 2024 Aug;76(4):219-231. doi: 10.1007/s00251-024-01345-9. Epub 2024 Jun 28. Immunogenetics. 2024. PMID: 38940861
-
Important denominator between autoimmune comorbidities: a review of class II HLA, autoimmune disease, and the gut.Front Immunol. 2023 Sep 26;14:1270488. doi: 10.3389/fimmu.2023.1270488. eCollection 2023. Front Immunol. 2023. PMID: 37828987 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

