Apolipoprotein E ε4 modulates astrocyte neuronal support functions in the presence of amyloid-β
- PMID: 36762973
- PMCID: PMC10903110
- DOI: 10.1111/jnc.15781
Apolipoprotein E ε4 modulates astrocyte neuronal support functions in the presence of amyloid-β
Abstract
Apolipoprotein E (APOE) is a lipid transporter produced predominantly by astrocytes in the brain. The ε4 variant of APOE (APOE4) is the strongest and most common genetic risk factor for Alzheimer's disease (AD). Although the molecular mechanisms of this increased risk are unclear, APOE4 is known to alter immune signaling and lipid and glucose metabolism. Astrocytes provide various forms of support to neurons, including regulating neuronal metabolism and immune responses through cytokine signaling. Changes in astrocyte function because of APOE4 may therefore decrease neuronal support, leaving neurons more vulnerable to stress and disease insults. To determine whether APOE4 alters astrocyte neuronal support functions, we measured glycolytic and oxidative metabolism of neurons treated with conditioned media from APOE4 or APOE3 (the common, risk-neutral variant) primary astrocyte cultures. We found that APOE4 neurons treated with conditioned media from resting APOE4 astrocytes had similar metabolism to APOE3 neurons treated with media from resting APOE3 astrocytes, but treatment with astrocytic conditioned media from astrocytes challenged with amyloid-β (Aβ), a key pathological protein in AD, caused APOE4 neurons to increase their basal mitochondrial and glycolytic metabolic rates more than APOE3 neurons. These changes were not because of differences in astrocytic lactate production or glucose utilization, but instead correlated with increased glycolytic ATP production and a lack of cytokine secretion in response to Aβ. Additionally, we identified that astrocytic cytokine signatures could predict basal metabolism of neurons treated with the astrocytic conditioned media. Together, these findings suggest that in the presence of Aβ, APOE4 astrocytes alter immune and metabolic functions that result in a compensatory increase in neuronal metabolic stress.
Keywords: ATP; amyloid-β; astrocytes; cytokines; glucose; glycolysis; immunometabolism.
© 2023 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interests with the content of this article.
Figures

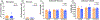



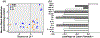
References
-
- Arnaud L, Benech P, Greetham L, Stephan D, Jimenez A, Jullien N, García-González L, Tsvetkov PO, Devred F, Sancho-Martinez I, Izpisua Belmonte JC, Baranger K, Rivera S, & Nivet E (2022). APOE4 drives inflammation in human astrocytes via TAGLN3 repression and NF-κB activation. Cell Reports, 40, 111200. - PubMed
-
- Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, Ben Haim L, AMH Y, Batiuk MY, Prakash K, Brown A, Roberts K, Paredes MF, Kawaguchi R, Stockley JH, Sabeur K, Chang SM, Huang E, Hutchinson P, … Rowitch DH (2020). Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nature Neuroscience, 23(4), 500–509. - PMC - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, & Malenka RC (2002). Control of synaptic strength by glial TNFα. Science, 1979(295), 2282–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

