In Vivo Screening Unveils Pervasive RNA-Binding Protein Dependencies in Leukemic Stem Cells and Identifies ELAVL1 as a Therapeutic Target
- PMID: 36763002
- PMCID: PMC10150294
- DOI: 10.1158/2643-3230.BCD-22-0086
In Vivo Screening Unveils Pervasive RNA-Binding Protein Dependencies in Leukemic Stem Cells and Identifies ELAVL1 as a Therapeutic Target
Abstract
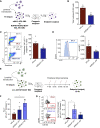
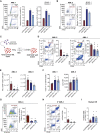
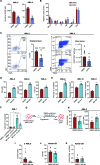
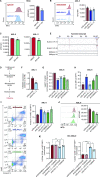
Acute myeloid leukemia (AML) is fueled by leukemic stem cells (LSC) whose determinants are challenging to discern from hematopoietic stem cells (HSC) or uncover by approaches focused on general cell properties. We have identified a set of RNA-binding proteins (RBP) selectively enriched in human AML LSCs. Using an in vivo two-step CRISPR-Cas9 screen to assay stem cell functionality, we found 32 RBPs essential for LSCs in MLL-AF9;NrasG12D AML. Loss-of-function approaches targeting key hit RBP ELAVL1 compromised LSC-driven in vivo leukemic reconstitution, and selectively depleted primitive malignant versus healthy cells. Integrative multiomics revealed differentiation, splicing, and mitochondrial metabolism as key features defining the leukemic ELAVL1-mRNA interactome with mitochondrial import protein, TOMM34, being a direct ELAVL1-stabilized target whose repression impairs AML propagation. Altogether, using a stem cell-adapted in vivo CRISPR screen, this work demonstrates pervasive reliance on RBPs as regulators of LSCs and highlights their potential as therapeutic targets in AML.
Significance: LSC-targeted therapies remain a significant unmet need in AML. We developed a stem-cell-adapted in vivo CRISPR screen to identify key LSC drivers. We uncover widespread RNA-binding protein dependencies in LSCs, including ELAVL1, which we identify as a novel therapeutic vulnerability through its regulation of mitochondrial metabolism. This article is highlighted in the In This Issue feature, p. 171.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
- 2643-3230. doi: 10.1158/2643-3230.BCD-4-3-ITI doi: 10.1158/2643-3230.BCD-4-3-ITI
References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52. - PubMed
-
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell 2014;14:275–91. - PubMed
-
- Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017;547:104–8. - PubMed
-
- Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001;98:2301–7. - PubMed
-
- Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood 2003;101:3142–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

