Structural mechanisms for the activation of human cardiac KCNQ1 channel by electro-mechanical coupling enhancers
- PMID: 36763058
- PMCID: PMC9661191
- DOI: 10.1073/pnas.2207067119
Structural mechanisms for the activation of human cardiac KCNQ1 channel by electro-mechanical coupling enhancers
Abstract
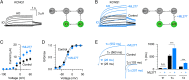
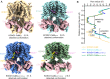
The cardiac KCNQ1 potassium channel carries the important IKs current and controls the heart rhythm. Hundreds of mutations in KCNQ1 can cause life-threatening cardiac arrhythmia. Although KCNQ1 structures have been recently resolved, the structural basis for the dynamic electro-mechanical coupling, also known as the voltage sensor domain-pore domain (VSD-PD) coupling, remains largely unknown. In this study, utilizing two VSD-PD coupling enhancers, namely, the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) and a small-molecule ML277, we determined 2.5-3.5 Å resolution cryo-electron microscopy structures of full-length human KCNQ1-calmodulin (CaM) complex in the apo closed, ML277-bound open, and ML277-PIP2-bound open states. ML277 binds at the "elbow" pocket above the S4-S5 linker and directly induces an upward movement of the S4-S5 linker and the opening of the activation gate without affecting the C-terminal domain (CTD) of KCNQ1. PIP2 binds at the cleft between the VSD and the PD and brings a large structural rearrangement of the CTD together with the CaM to activate the PD. These findings not only elucidate the structural basis for the dynamic VSD-PD coupling process during KCNQ1 gating but also pave the way to develop new therapeutics for anti-arrhythmia.
Keywords: E-M coupling; KCNQ1; ML277; PIP2.
Conflict of interest statement
The authors declare no competing interest.
Figures
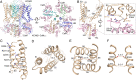




Similar articles
-
The membrane electric field regulates the PIP2-binding site to gate the KCNQ1 channel.Proc Natl Acad Sci U S A. 2023 May 23;120(21):e2301985120. doi: 10.1073/pnas.2301985120. Epub 2023 May 16. Proc Natl Acad Sci U S A. 2023. PMID: 37192161 Free PMC article.
-
ML277 specifically enhances the fully activated open state of KCNQ1 by modulating VSD-pore coupling.Elife. 2019 Jul 22;8:e48576. doi: 10.7554/eLife.48576. Elife. 2019. PMID: 31329101 Free PMC article.
-
Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome.Cell. 2017 Jun 1;169(6):1042-1050.e9. doi: 10.1016/j.cell.2017.05.019. Cell. 2017. PMID: 28575668 Free PMC article.
-
Gating and Regulation of KCNQ1 and KCNQ1 + KCNE1 Channel Complexes.Front Physiol. 2020 Jun 4;11:504. doi: 10.3389/fphys.2020.00504. eCollection 2020. Front Physiol. 2020. PMID: 32581825 Free PMC article. Review.
-
The membrane protein KCNQ1 potassium ion channel: Functional diversity and current structural insights.Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183148. doi: 10.1016/j.bbamem.2019.183148. Epub 2019 Dec 9. Biochim Biophys Acta Biomembr. 2020. PMID: 31825788 Free PMC article. Review.
Cited by
-
Mechanisms of KCNQ1 gating modulation by KCNE1/3 for cell-specific function.Cell Res. 2025 Jul 31. doi: 10.1038/s41422-025-01152-1. Online ahead of print. Cell Res. 2025. PMID: 40745202
-
Constitutive opening of the Kv7.2 pore activation gate causes KCNQ2-developmental encephalopathy.Proc Natl Acad Sci U S A. 2024 Dec 3;121(49):e2412388121. doi: 10.1073/pnas.2412388121. Epub 2024 Nov 27. Proc Natl Acad Sci U S A. 2024. PMID: 39602259 Free PMC article.
-
Progress in understanding the regulatory mechanisms of immune checkpoint proteins PD-1 and PD-L1 expression.Clin Transl Oncol. 2025 Aug;27(8):3261-3271. doi: 10.1007/s12094-024-03835-4. Epub 2025 Jan 8. Clin Transl Oncol. 2025. PMID: 39776397 Review.
-
KCNQ1 p.D446E Variant as a Risk Allele for Arrhythmogenic Phenotypes: Electrophysiological Characterization Reveals a Complex Phenotype Affecting the Slow Delayed Rectifier Potassium Current (IKs) Voltage Dependence by Causing a Hyperpolarizing Shift and a Lack of Response to Protein Kinase A Activation.Int J Mol Sci. 2024 Jan 12;25(2):953. doi: 10.3390/ijms25020953. Int J Mol Sci. 2024. PMID: 38256028 Free PMC article.
-
Adipose stem cell exosomes, stimulated by pro-inflammatory factors, enhance immune evasion in triple-negative breast cancer by modulating the HDAC6/STAT3/PD-L1 pathway through the transporter UCHL1.Cancer Cell Int. 2024 Nov 20;24(1):385. doi: 10.1186/s12935-024-03557-1. Cancer Cell Int. 2024. PMID: 39568023 Free PMC article.
References
-
- Wang Q., et al. , Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat. Genet. 12, 17–23 (1996). - PubMed
-
- Sanguinetti M. C., et al. , Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384, 80–83 (1996). - PubMed
-
- Barhanin J., et al. , K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384, 78–80 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

