Structural mechanisms for the activation of human cardiac KCNQ1 channel by electro-mechanical coupling enhancers
- PMID: 36763058
- PMCID: PMC9661191
- DOI: 10.1073/pnas.2207067119
Structural mechanisms for the activation of human cardiac KCNQ1 channel by electro-mechanical coupling enhancers
Abstract
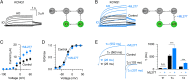
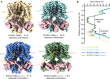
The cardiac KCNQ1 potassium channel carries the important IKs current and controls the heart rhythm. Hundreds of mutations in KCNQ1 can cause life-threatening cardiac arrhythmia. Although KCNQ1 structures have been recently resolved, the structural basis for the dynamic electro-mechanical coupling, also known as the voltage sensor domain-pore domain (VSD-PD) coupling, remains largely unknown. In this study, utilizing two VSD-PD coupling enhancers, namely, the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) and a small-molecule ML277, we determined 2.5-3.5 Å resolution cryo-electron microscopy structures of full-length human KCNQ1-calmodulin (CaM) complex in the apo closed, ML277-bound open, and ML277-PIP2-bound open states. ML277 binds at the "elbow" pocket above the S4-S5 linker and directly induces an upward movement of the S4-S5 linker and the opening of the activation gate without affecting the C-terminal domain (CTD) of KCNQ1. PIP2 binds at the cleft between the VSD and the PD and brings a large structural rearrangement of the CTD together with the CaM to activate the PD. These findings not only elucidate the structural basis for the dynamic VSD-PD coupling process during KCNQ1 gating but also pave the way to develop new therapeutics for anti-arrhythmia.
Keywords: E-M coupling; KCNQ1; ML277; PIP2.
Conflict of interest statement
The authors declare no competing interest.
Figures
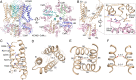




References
-
- Wang Q., et al. , Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat. Genet. 12, 17–23 (1996). - PubMed
-
- Sanguinetti M. C., et al. , Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384, 80–83 (1996). - PubMed
-
- Barhanin J., et al. , K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384, 78–80 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

