Multifocal electroretinography increases following experimental glaucoma in nonhuman primates with retinal ganglion cell axotomy
- PMID: 36763214
- PMCID: PMC10284020
- DOI: 10.1007/s10633-023-09922-1
Multifocal electroretinography increases following experimental glaucoma in nonhuman primates with retinal ganglion cell axotomy
Abstract
Purpose: To determine whether short-latency changes in multifocal electroretinography (mfERG) observed in experimental glaucoma (EG) are secondary solely to retinal ganglion cell (RGC) loss or whether there is a separate contribution from elevated intraocular pressure (IOP).
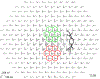
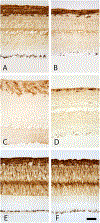
Methods: Prior to operative procedures, a series of baseline mfERGs were recorded from six rhesus macaques using a 241-element unstretched stimulus. Animals then underwent hemiretinal endodiathermy axotomy (HEA) by placing burns along the inferior 180° of the optic nerve margin in the right eye (OD). mfERG recordings were obtained in each animal at regular intervals following for 3-4 months to allow stabilization of the HEA effects. Laser trabecular meshwork destruction (LTD) to elevate IOP was then performed; first-order kernel (K1) waveform root-mean-square (RMS) amplitudes for the short-latency segment of the mfERG wave (9-35 ms) were computed for two 7-hexagon groupings-the first located within the superior (non-axotomized) macula and the second within the inferior (axotomized) macula. Immunohistochemistry for glial fibrillary acidic protein (GFAP) was done.
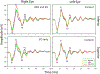
Results: By 3 months post HEA, there was marked thinning of the inferior nerve fiber layer as measured by optical coherence tomography. Compared with baseline, no statistically significant changes in 9-35 ms K1 RMS amplitudes were evident in either the axotomized or non-axotomized portions of the macula. Following LTD, mean IOP in HEA eyes rose to 46 ± 9 compared with 20 ± 2 mmHg (SD) in the fellow control eyes. In the HEA + EG eyes, statistically significant increases in K1 RMS amplitude were present in both the axotomized inferior and non-axotomized superior portions of the OD retinas. No changes in K1 RMS amplitude were found in the fellow control eyes from baseline to HEA epoch, but there was a smaller increase from baseline to HEA + EG. Upregulation of GFAP in the Müller cells was evident in both non-axotomized and axotomized retina in eyes with elevated IOP.
Conclusions: The RMS amplitudes of the short-latency mfERG K1 waveforms are not altered following axotomy but undergo marked increases following elevated IOP. This suggests that the increase in mfERG amplitude was not solely a result of RGC loss and may reflect photoreceptor and bipolar cell dysfunction and/or changes in Müller cells.
Keywords: Axotomy; Experimental glaucoma; Multifocal electroretinography; Supranormality.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Disclosure of potential conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures





References
-
- Kendell KR, Quigley HA, Kerrigan LA, Pease ME, Quigley EN. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995;36(1):200–5. Epub 1995/01/01. - PubMed
-
- Pelzel HR, Schlamp CL, Poulsen GL, Ver Hoeve JA, Nork TM, Nickells RW. Decrease of cone opsin mRNA in experimental ocular hypertension. Mol Vis. 2006;12:1272–82. Epub 20061026. - PubMed
-
- Harwerth RS, Smith EL 3rd, DeSantis L. Experimental glaucoma: perimetric field defects and intraocular pressure. J Glaucoma. 1997;6(6):390–401. Epub 1998/01/04. - PubMed
-
- Harwerth RS, Carter-Dawson L, Shen F, Smith EL 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40(10):2242–50. Epub 1999/09/07. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

