Efficacy and Safety of Ertugliflozin Added to Metformin: A Pooled Population from Asia with Type 2 Diabetes and Overweight or Obesity
- PMID: 36763328
- PMCID: PMC9944172
- DOI: 10.1007/s13300-022-01345-6
Efficacy and Safety of Ertugliflozin Added to Metformin: A Pooled Population from Asia with Type 2 Diabetes and Overweight or Obesity
Abstract
Introduction: The efficacy and safety of ertugliflozin have not been well characterized in Asian populations with type 2 diabetes (T2D) and overweight or obesity as defined by the Chinese Diabetes Society [body mass index (BMI) ≥ 24 kg/m2].
Methods: These post hoc analyses of pooled data from two randomized, double-blind, 26-week studies assessed the efficacy and safety of ertugliflozin (5 mg or 15 mg) compared with placebo in participants from Asia with T2D and baseline BMI ≥ 24 kg/m2, with inadequate glycemic control on metformin. Longitudinal analyses were used to calculate least squares (LS) mean [95% confidence interval (CI)] change from baseline in glycemic indices and body weight. The proportions of participants achieving efficacy targets and experiencing adverse events (AEs) were assessed.
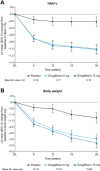
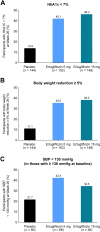
Results: The 445 participants had a mean age of 55.5 years, T2D duration 6.6 years, glycated hemoglobin (HbA1c) 8.1%, and BMI 27.6 kg/m2. At week 26, placebo-adjusted LS mean (95% CI) changes from baseline for ertugliflozin 5 mg and 15 mg, respectively, were - 0.78% (- 0.95% to - 0.61%) and - 0.80% (- 0.98% to - 0.63%) for HbA1c, and - 1.74 kg (- 2.29 kg to - 1.19 kg) and - 2.04 kg (- 2.60 kg to - 1.48 kg) for body weight. A greater proportion of participants receiving ertugliflozin 5 mg and 15 mg versus placebo, respectively, achieved HbA1c < 7.0% (42.1% and 46.3% vs. 13.9%), body weight reduction ≥ 5% (35.5% and 38.3% vs. 11.1%), and systolic blood pressure < 130 mmHg (42.4% and 34.5% vs. 21.7%). The proportion of participants with AEs was 52.6% (ertugliflozin 5 mg), 52.3% (ertugliflozin 15 mg), and 55.6% (placebo).
Conclusions: In participants from Asia with T2D inadequately controlled by metformin monotherapy, and BMI ≥24 kg/m2, ertugliflozin (5 mg or 15 mg) resulted in greater glycemic and body weight reductions compared with placebo and was generally well tolerated.
Trial registration: Clinicaltrials.gov identifiers NCT02033889, NCT02630706.
Keywords: Asia; Ertugliflozin; Obese; Overweight; Type 2 diabetes.
© 2023. Pfizer Inc., Merck & Co., Inc., Rahway, NJ, USA and its affiliates, and Linong Ji.
Figures



References
-
- Safiri S, Karamzad N, Kaufman JS, et al. Prevalence, deaths and disability-adjusted-life-years (DALYs) due to type 2 diabetes and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. Front Endocrinol (Lausanne) 2022;13:838027. doi: 10.3389/fendo.2022.838027. - DOI - PMC - PubMed
-
- Ruan Y, Yan QH, Xu JY, et al. Epidemiology of diabetes in adults aged 35 and older from Shanghai, China. Biomed Environ Sci. 2016;29:408–416. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

