Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches
- PMID: 36763330
- PMCID: PMC9913034
- DOI: 10.1007/s13346-023-01290-2
Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches
Abstract
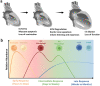
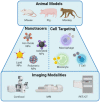
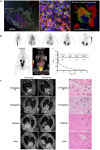
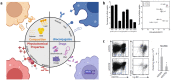
Ischemic heart failure (IHF) is a leading cause of morbidity and mortality worldwide, for which heart transplantation remains the only definitive treatment. IHF manifests from myocardial infarction (MI) that initiates tissue remodeling processes, mediated by mechanical changes in the tissue (loss of contractility, softening of the myocardium) that are interdependent with cellular mechanisms (cardiomyocyte death, inflammatory response). The early remodeling phase is characterized by robust inflammation that is necessary for tissue debridement and the initiation of repair processes. While later transition toward an immunoregenerative function is desirable, functional reorientation from an inflammatory to reparatory environment is often lacking, trapping the heart in a chronically inflamed state that perpetuates cardiomyocyte death, ventricular dilatation, excess fibrosis, and progressive IHF. Therapies can redirect the immune microenvironment, including biotherapeutic and biomaterial-based approaches. In this review, we outline these existing approaches, with a particular focus on the immunomodulatory effects of therapeutics (small molecule drugs, biomolecules, and cell or cell-derived products). Cardioprotective strategies, often focusing on immunosuppression, have shown promise in pre-clinical and clinical trials. However, immunoregenerative therapies are emerging that often benefit from exacerbating early inflammation. Biomaterials can be used to enhance these therapies as a result of their intrinsic immunomodulatory properties, parallel mechanisms of action (e.g., mechanical restraint), or by enabling cell or tissue-targeted delivery. We further discuss translatability and the continued progress of technologies and procedures that contribute to the bench-to-bedside development of these critically needed treatments.
Keywords: Biomaterials; Biotherapeutics; Heart failure; Immune modulation; Inflammatory disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143(8). - PubMed
-
- Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The lancet. 2016;388(10053):1659–1724. - PMC - PubMed
-
- GóMez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction. Circulation. 2001;104(6):688–693. - PubMed
-
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

