Effect of Rivaroxaban vs Enoxaparin on Major Cardiac Adverse Events and Bleeding Risk in the Acute Phase of Acute Coronary Syndrome: The H-REPLACE Randomized Equivalence and Noninferiority Trial
- PMID: 36763358
- PMCID: PMC9918885
- DOI: 10.1001/jamanetworkopen.2022.55709
Effect of Rivaroxaban vs Enoxaparin on Major Cardiac Adverse Events and Bleeding Risk in the Acute Phase of Acute Coronary Syndrome: The H-REPLACE Randomized Equivalence and Noninferiority Trial
Abstract
Importance: Parenteral enoxaparin is a preferred anticoagulant used in the acute phase for patients with acute coronary syndrome (ACS). The safety and efficacy of short-term low-dose rivaroxaban in this clinical setting remain unknown.
Objective: To compare the safety and efficacy of rivaroxaban vs enoxaparin in the acute phase of ACS.
Design, setting, and participants: This multicenter, prospective, open-label, active-controlled, equivalence and noninferiority trial was conducted from January 2017 through May 2021 with a 6-month follow-up at 21 hospitals in China. Participants included patients with ACS missing the primary reperfusion window or before selective revascularization. Data were analyzed from November 2021 to November 2022.
Interventions: Participants were randomized 1:1:1 to oral rivaroxaban 2.5 mg or 5 mg or 1 mg/kg subcutaneous enoxaparin twice daily in addition to dual antiplatelet therapy (DAPT; aspirin 100 mg and clopidogrel 75 mg once daily) for a mean of 3.7 days.
Main outcomes and measures: The primary safety end point was bleeding events, as defined by the International Society on Thrombosis and Haemostasis, and the primary efficacy end point was major adverse cardiovascular events (MACEs), including cardiac death, myocardial infarction, rerevascularization, or stroke during the 6-month follow-up.
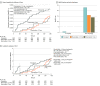
Results: Of 2055 enrolled patients, 2046 (99.6%) completed the trial (mean [SD] age 65.8 [8.2] years, 1443 [70.5%] male) and were randomized to enoxaparin (680 patients), rivaroxaban 2.5 mg (683 patients), or rivaroxaban 5 mg (683 patients). Bleeding rates were 46 patients (6.8%) in the enoxaparin group, 32 patients (4.7%) in the rivaroxaban 2.5 mg group, and 36 patients (5.3%)in the rivaroxaban 5 mg group (rivaroxaban 2.5 mg vs enoxaparin: noninferiority hazard ratio [HR], 0.68; 95% CI, 0.43 to 1.07; P = .005; rivaroxaban 5 mg vs enoxaparin: noninferiority HR, 0.88; 95% CI, 0.70 to 1.09; P = .001). The incidence of MACEs was similar among groups, and noninferiority was reached in the rivaroxaban 5 mg group (HR, 0.60; 95% CI, 0.31 to 1.16, P = .02) but not in the rivaroxaban 2.5 mg group (HR, 0.68; 95% CI, 0.36 to 1.30; P = .05) compared with the enoxaparin group.
Conclusions and relevance: In this equivalence and noninferiority trial, oral rivaroxaban 5 mg showed noninferiority to subcutaneous enoxaparin (1 mg/kg) for patients with ACS treated with DAPT during the acute phase. Results of this feasibility study provide useful information for designing future randomized clinical trials with sufficient sample sizes.
Trial registration: ClinicalTrials.gov Identifier: NCT03363035.
Conflict of interest statement
Figures

Comment in
-
Rivaroxaban for Patients With Acute Coronary Syndromes-Where Do We Stand?JAMA Netw Open. 2023 Feb 1;6(2):e2255724. doi: 10.1001/jamanetworkopen.2022.55724. JAMA Netw Open. 2023. PMID: 36763365 No abstract available.
References
-
- Ricciardi MJ, Selzer F, Marroquin OC, et al. . Incidence and predictors of 30-day hospital readmission rate following percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol. 2012;110(10):1389-1396. doi:10.1016/j.amjcard.2012.07.002 - DOI - PMC - PubMed
-
- Song F, Yu M, Yang J, et al. ; China Acute Myocardial Infarction (CAMI) Registry study group . Symptom-onset-to-balloon time, ST-segment resolution and in-hospital mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention in china: from China Acute Myocardial Infarction Registry. Am J Cardiol. 2016;118(9):1334-1339. doi:10.1016/j.amjcard.2016.07.058 - DOI - PubMed
-
- Cohen M, Demers C, Gurfinkel EP, et al. ; Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group . A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. N Engl J Med. 1997;337(7):447-452. doi:10.1056/NEJM199708143370702 - DOI - PubMed
-
- Goodman SG, Cohen M, Bigonzi F, et al. . Randomized trial of low molecular weight heparin (enoxaparin) versus unfractionated heparin for unstable coronary artery disease: one-year results of the ESSENCE Study: efficacy and safety of subcutaneous enoxaparin in non–Q wave coronary events. J Am Coll Cardiol. 2000;36(3):693-698. doi:10.1016/S0735-1097(00)00808-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

