Combining arterial blood contrast with BOLD increases fMRI intracortical contrast
- PMID: 36763562
- PMCID: PMC10028680
- DOI: 10.1002/hbm.26227
Combining arterial blood contrast with BOLD increases fMRI intracortical contrast
Abstract
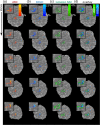
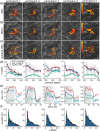
BOLD fMRI is widely applied in human neuroscience but is limited in its spatial specificity due to a cortical-depth-dependent venous bias. This reduces its localization specificity with respect to neuronal responses, a disadvantage for neuroscientific research. Here, we modified a submillimeter BOLD protocol to selectively reduce venous and tissue signal and increase cerebral blood volume weighting through a pulsed saturation scheme (dubbed Arterial Blood Contrast) at 7 T. Adding Arterial Blood Contrast on top of the existing BOLD contrast modulated the intracortical contrast. Isolating the Arterial Blood Contrast showed a response free of pial-surface bias. The results suggest that Arterial Blood Contrast can modulate the typical fMRI spatial specificity, with important applications in in-vivo neuroscience.
Keywords: 3D-EPI; arterial blood contrast; cerebral blood volume; layer-dependent fMRI; magnetization prepared fMRI; ultrahigh field MRI.
© 2023 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

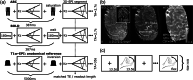
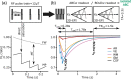

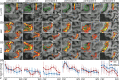
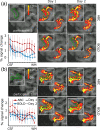

References
-
- Bottomley, P. A. , & Edelstein, W. A. (1981). Power deposition in whole‐body NMR imaging. Medical Physics, 8, 510–512. - PubMed
-
- Boxerman, J. L. , Bandettini, P. A. , Kwong, K. K. , Baker, J. R. , Davis, T. L. , Rosen, B. R. , & Weisskoff, R. M. (1995). The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion‐weighted studies in vivo. Magnetic Resonance in Medicine, 34, 4–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

