Real-world utilization of SARS-CoV-2 serological testing in RNA positive patients across the United States
- PMID: 36763574
- PMCID: PMC9916659
- DOI: 10.1371/journal.pone.0281365
Real-world utilization of SARS-CoV-2 serological testing in RNA positive patients across the United States
Abstract
Background: As diagnostic tests for COVID-19 were broadly deployed under Emergency Use Authorization, there emerged a need to understand the real-world utilization and performance of serological testing across the United States.
Methods: Six health systems contributed electronic health records and/or claims data, jointly developed a master protocol, and used it to execute the analysis in parallel. We used descriptive statistics to examine demographic, clinical, and geographic characteristics of serology testing among patients with RNA positive for SARS-CoV-2.
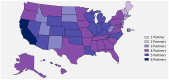
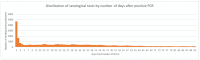
Results: Across datasets, we observed 930,669 individuals with positive RNA for SARS-CoV-2. Of these, 35,806 (4%) were serotested within 90 days; 15% of which occurred <14 days from the RNA positive test. The proportion of people with a history of cardiovascular disease, obesity, chronic lung, or kidney disease; or presenting with shortness of breath or pneumonia appeared higher among those serotested compared to those who were not. Even in a population of people with active infection, race/ethnicity data were largely missing (>30%) in some datasets-limiting our ability to examine differences in serological testing by race. In datasets where race/ethnicity information was available, we observed a greater distribution of White individuals among those serotested; however, the time between RNA and serology tests appeared shorter in Black compared to White individuals. Test manufacturer data was available in half of the datasets contributing to the analysis.
Conclusion: Our results inform the underlying context of serotesting during the first year of the COVID-19 pandemic and differences observed between claims and EHR data sources-a critical first step to understanding the real-world accuracy of serological tests. Incomplete reporting of race/ethnicity data and a limited ability to link test manufacturer data, lab results, and clinical data challenge the ability to assess the real-world performance of SARS-CoV-2 tests in different contexts and the overall U.S. response to current and future disease pandemics.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Natalie E Sheils is an employee of Optum Labs and owns stock in the parent company UnitedHealth group. Anthony M Louder is a paid employee of Aetion and hold Aetion stock options. Nancy D. Lin was an employee of Health Catalyst at the time the work was performed. Jennifer Gatz is a full-time employee of Regenstrief Institute, which provides independent research services to entities including those within the pharmaceutical and medical device industries. Shaun J. Grannis serves as Chief Medical Information Officer for the Indiana Health Information Exchange, and is a founding partner of Uppstroms, LLC. Carly Kabelac is a paid employee of Aetion and hold Aetion stock options. Carrie L. Byington has intellectual property in and receives royalties from BioFire, Inc. She serves as a scientific advisor to IDbyDNA (San Francisco, CA and Salt Lake City, UT). Dr. Byington is on the Board of the Commonwealth Fund. Atul J. Butte is a co-founder and consultant to Personalis and NuMedii; consultant to Samsung, Mango Tree Corporation, and in the recent past, 10x Genomics, Helix, Pathway Genomics, and Verinata (Illumina); has served on paid advisory panels or boards for Geisinger Health, Regenstrief Institute, Gerson Lehman Group, AlphaSights, Covance, Novartis, Genentech, Merck, and Roche; is a shareholder in Personalis and NuMedii; is a minor shareholder in Apple, Facebook, Alphabet (Google), Microsoft, Amazon, Snap, Snowflake, 10x Genomics, Illumina, Nuna Health, Assay Depot (Scientist.com), Vet24seven, Regeneron, Sanofi, Royalty Pharma, Pfizer, BioNTech, AstraZeneca, Moderna, Biogen, Twist Bioscience, Pacific Biosciences, Editas Medicine, Invitae, Doximity, and Sutro, and several other non-health related companies and mutual funds; and has received honoraria and travel reimbursement for invited talks from Johnson and Johnson, Roche, Genentech, Pfizer, Merck, Lilly, Takeda, Varian, Mars, Siemens, Optum, Abbott, Celgene, AstraZeneca, AbbVie, Westat, several investment and venture capital firms, and many academic institutions, medical or disease specific foundations and associations, and health systems. Atul Butte receives royalty payments through Stanford University, for several patents and other disclosures licensed to NuMedii and Personalis. Carla Rodriguez-Watson receives research support from Merck, Novartis, Pfizer, Lilly, Janssen, and AbbVie. She holds minor stock in Gilead.
Figures

References
-
- Lassaunière R, Frische A, Harboe ZB, Nielsen AC, Fomsgaard A, Krogfelt KA, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. MedRxiv. 2020.
-
- Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, et al. Test performance evaluation of SARS-CoV-2 serological assays. MedRxiv. 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

