An in vitro carcinogenesis model for cervical cancer harboring episomal form of HPV16
- PMID: 36763589
- PMCID: PMC9916646
- DOI: 10.1371/journal.pone.0281069
An in vitro carcinogenesis model for cervical cancer harboring episomal form of HPV16
Abstract
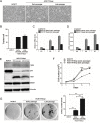
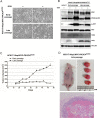
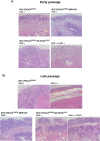
Deregulated expression of viral E6 and E7 genes often caused by viral genome integration of high-risk human papillomaviruses (HR-HPVs) into host DNA and additional host genetic alterations are thought to be required for the development of cervical cancer. However, approximately 15% of invasive cervical cancer specimens contain only episomal HPV genomes. In this study, we investigated the tumorigenic potential of human cervical keratinocytes harboring only the episomal form of HPV16 (HCK1T/16epi). We found that the HPV16 episomal form is sufficient for promoting cell proliferation and colony formation of parental HCK1T cells. Ectopic expression of host oncogenes, MYC and PIK3CAE545K, enhanced clonogenic growth of both early- and late-passage HCK1T/16epi cells, but conferred tumor-initiating ability only to late-passage HCK1T/16epi cells. Interestingly, the expression levels of E6 and E7 were rather lower in late-passage than in early-passage cells. Moreover, additional introduction of a constitutively active MEK1 (MEK1DD) and/or KRASG12V into HCK1T/16epi cells resulted in generation of highly potent tumor-initiating cells. Thus an in vitro model for progression of cervical neoplasia with episomal HPV16 was established. In the model, constitutively active mutation of PIK3CA, PIK3CAE545K, and overexpression of MYC, in the cells with episomal HPV16 genome were not sufficient, but an additional event such as activation of the RAS-MEK pathway was required for progression to tumorigenicity.
Copyright: © 2023 Wongjampa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

