Fertility Preservation Practices at Pediatric Oncology Institutions in the United States: A Report From the Children's Oncology Group
- PMID: 36763922
- PMCID: PMC10113112
- DOI: 10.1200/OP.22.00349
Fertility Preservation Practices at Pediatric Oncology Institutions in the United States: A Report From the Children's Oncology Group
Abstract
Purpose: Fertility discussions are an integral part of comprehensive care for pediatric, adolescent, and young adult patients newly diagnosed with cancer and are supported by national guidelines. Current institutional practices are poorly understood.
Methods: A cross-sectional survey was distributed to 220 Children's Oncology Group member institutions regarding fertility discussion practices. Descriptive statistics were calculated for all variables. The association between specific practices and selected outcomes on the basis of sex was examined via multivariable logistic regression.
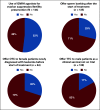
Results: One hundred forty-four programs (65.5%) returned surveys. Of these, 65 (45.1%) reported routine discussions of fertility with all female patients and 55 (38.5%) all male patients (P = .25). Ninety-two (63.8%) reported no specific criteria for offering females fertility preservation (FP), compared with 40 (27.7%) for males (P < .001). Program characteristics associated with fertility discussions included reproductive endocrinology and infertility on site (females odds ratio [OR], 2.1; 95% CI, 1.0 to 4.3), discussion documentation mandate (females OR, 2.3; 95% CI, 1.0 to 5.5; males OR, 3.5; 95% CI, 1.4 to 8.7), and cumulative institution-based FP infrastructure (which included [1] routine practice of documentation, [2] template for documentation, [3] mandate for documentation, and [4] availability of FP navigation; females OR, 1.6; 95% CI, 1.1 to 2.3; males OR, 2.3; 95% CI, 1.6 to 3.4). Utilization of practices unsupported by guidelines included offering sperm banking after treatment initiation (39/135 programs; 28.9%), gonadotropin-releasing hormone analogs for ovarian suppression/FP (75/144 programs; 52.1%), ovarian tissue cryopreservation at diagnosis for patients with leukemia (19/64 programs; 29.7%), and testicular tissue cryopreservation (23/138 programs; 16.7%) not part of a clinical trial.
Conclusion: Despite recommended guidelines, fertility discussions with patients/families before treatment initiation are not routine at Children's Oncology Group institutions. Standard criteria to determine which options should be offered to patients are more common for males than females.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
Similar articles
-
Infrastructure of Fertility Preservation Services for Pediatric Cancer Patients: A Report From the Children's Oncology Group.JCO Oncol Pract. 2022 Mar;18(3):e325-e333. doi: 10.1200/OP.21.00275. Epub 2021 Oct 28. JCO Oncol Pract. 2022. PMID: 34709943 Free PMC article.
-
Fertility preservation before and after cancer treatment in children, adolescents, and young adults.Cancer. 2024 Feb 1;130(3):344-355. doi: 10.1002/cncr.35108. Epub 2023 Nov 14. Cancer. 2024. PMID: 37962199 Free PMC article. Review.
-
Fertility Preservation Counseling for Pediatric and Adolescent Cancer Patients.J Adolesc Young Adult Oncol. 2016 Mar;5(1):58-63. doi: 10.1089/jayao.2015.0040. Epub 2015 Nov 30. J Adolesc Young Adult Oncol. 2016. PMID: 26812454
-
Patient factors associated with sperm cryopreservation among at-risk adolescents newly diagnosed with cancer.Cancer. 2018 Sep 1;124(17):3567-3575. doi: 10.1002/cncr.31596. Epub 2018 Jul 5. Cancer. 2018. PMID: 29975417 Free PMC article.
-
Fertility preservation for pediatric patients with hemoglobinopathies: Multidisciplinary counseling needed to optimize outcomes.Front Endocrinol (Lausanne). 2022 Oct 24;13:985525. doi: 10.3389/fendo.2022.985525. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36353243 Free PMC article. Review.
Cited by
-
Young People with Cancer: A Growing Population in Need of United Attention.Curr Oncol Rep. 2025 Apr;27(4):333-335. doi: 10.1007/s11912-025-01661-7. Epub 2025 Mar 19. Curr Oncol Rep. 2025. PMID: 40106216 Free PMC article. Review.
-
Thawing fertility: a view of ovarian tissue cryopreservation processes and review of ovarian transplant research.Fertil Steril. 2024 Oct;122(4):574-585. doi: 10.1016/j.fertnstert.2024.07.005. Epub 2024 Jul 9. Fertil Steril. 2024. PMID: 38992745 Review.
-
Impact of the Children's Oncology Group's supportive care clinical practice guideline endorsement program: An institutional survey.Pediatr Blood Cancer. 2024 Oct;71(10):e31178. doi: 10.1002/pbc.31178. Epub 2024 Jul 15. Pediatr Blood Cancer. 2024. PMID: 39010277
-
Fertility Potential and Gonadal Function in Survivors of Reduced-Intensity Hematopoietic Stem Cell Transplantation.Transplant Cell Ther. 2024 May;30(5):534.e1-534.e13. doi: 10.1016/j.jtct.2024.02.002. Epub 2024 Feb 9. Transplant Cell Ther. 2024. PMID: 38342136 Free PMC article.
-
Clinical Practice Guideline-Inconsistent Management of Fertility Preservation in Pediatric Cancer Patients in Community Settings: A Children's Oncology Group Study.J Adolesc Young Adult Oncol. 2024 Oct;13(5):776-784. doi: 10.1089/jayao.2024.0022. Epub 2024 May 21. J Adolesc Young Adult Oncol. 2024. PMID: 38770790
References
-
- Coccia PF, Pappo AS, Beaupin L, et al. : Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:66-97, 2018 - PubMed
-
- Practice Committee of the American Society for Reproductive Medicine : Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril 112:1022-1033, 2019 - PubMed
-
- Klipstein S, Fallat ME, Savelli S, et al. : Fertility preservation for pediatric and adolescent patients with cancer: Medical and ethical considerations. Pediatrics 145:e20193994, 2020 - PubMed
-
- Oktay K, Harvey BE, Partridge AH, et al. : Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 36:1994-2001, 2018 - PubMed
-
- Algarroba GN, Sanfilippo JS, Valli-Pulaski H: Female fertility preservation in the pediatric and adolescent cancer patient population. Best Pract Res Clin Obstet Gynaecol 48:147-157, 2018 - PubMed