Smooth muscle contribution to vaginal viscoelastic response
- PMID: 36764168
- PMCID: PMC10339229
- DOI: 10.1016/j.jmbbm.2023.105702
Smooth muscle contribution to vaginal viscoelastic response
Abstract
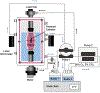
Smooth muscle cells contribute to the mechanical function of various soft tissues, however, their contribution to the viscoelastic response when subjected to multiaxial loading remains unknown. The vagina is a fibromuscular viscoelastic organ that is exposed to prolonged and increased pressures with daily activities and physiologic processes such as vaginal birth. The vagina changes in geometry over time under prolonged pressure, known as creep. Vaginal smooth muscle cells may contribute to creep. This may be critical for the function of vaginal and other soft tissues that experience fluctuations in their biomechanical environment. Therefore, the objective of this study was to develop methods to evaluate the contribution of smooth muscle to vaginal creep under multiaxial loading using extension - inflation tests. The vaginas from wildtype mice (C57BL/6 × 129SvEv; 3-6 months; n = 10) were stimulated with various concentrations of potassium chloride then subjected to the measured in vivo pressure (7 mmHg) for 100 s. In a different cohort of mice (n = 5), the vagina was stimulated with a single concentration of potassium chloride then subjected to 5 and 15 mmHg. A laser micrometer measured vaginal outer diameter in real-time. Immunofluorescence evaluated the expression of alpha-smooth muscle actin and myosin heavy chain in the vaginal muscularis (n = 6). When smooth muscle contraction was activated, vaginal creep behavior increased compared to the relaxed state. However, increased pressure decreased the active creep response. This study demonstrated that extension - inflation protocols can be used to evaluate smooth muscle contribution to the viscoelastic response of tubular soft tissues.
Keywords: Creep; Immunofluorescence; Smooth muscle cells; Vagina; Viscoelastic.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


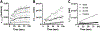


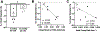


References
-
- Asundi KR, Rempel DM, 2008. MMP-1, IL-1 beta, and COX-2 mRNA expression is modulated by static load in rabbit flexor tendons. Ann. Biomed. Eng 36 (2), 237–243. - PubMed
-
- Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA, 2002. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am. J. Obstet. Gynecol 187 (1), 56–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

