STC2 activates PRMT5 to induce radioresistance through DNA damage repair and ferroptosis pathways in esophageal squamous cell carcinoma
- PMID: 36764215
- PMCID: PMC9929488
- DOI: 10.1016/j.redox.2023.102626
STC2 activates PRMT5 to induce radioresistance through DNA damage repair and ferroptosis pathways in esophageal squamous cell carcinoma
Abstract
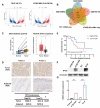
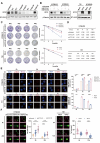
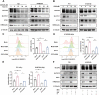
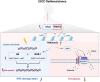
Radioresistance is the major reason for the failure of radiotherapy in esophageal squamous cell carcinoma (ESCC). Previous evidence indicated that stanniocalcin 2 (STC2) participates in various biological processes of malignant tumors. However, researches on its effect on radioresistance in cancers are limited. In this study, STC2 was screened out by RNA-sequencing and bioinformatics analyses as a potential prognosis predictor of ESCC radiosensitivity and then was determined to facilitate radioresistance. We found that STC2 expression is increased in ESCC tissues compared to adjacent normal tissues, and a higher level of STC2 is associated with poor prognosis. Also, STC2 mRNA and protein expression levels were higher in radioresistant cells than in their parental cells. Further investigation revealed that STC2 could interact with protein methyltransferase 5 (PRMT5) and activate PRMT5, thus leading to the increased expression of symmetric dimethylation of histone H4 on Arg 3 (H4R3me2s). Mechanistically, STC2 can promote DDR through the homologous recombination and non-homologous end joining pathways by activating PRMT5. Meanwhile, STC2 can participate in SLC7A11-mediated ferroptosis in a PRMT5-dependent manner. Finally, these results were validated through in vivo experiments. These findings uncovered that STC2 might be an attractive therapeutic target to overcome ESCC radioresistance.
Keywords: DNA damage Repair; Esophageal squamous cell carcinoma; Ferroptosis; PRMT5; Radioresistance; STC2.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures









References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016;66:115–132. - PubMed
-
- Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W., Li X., Wang L., Wang L., Liu Y., Liu J., Zhang M., Qi J., Yu S., Afshin A., Gakidou E., Glenn S., Krish V.S., Miller-Petrie M.K., Mountjoy-Venning W.C., Mullany E.C., Redford S.B., Liu H., Naghavi M., Hay S.I., Wang L., Murray C.J.L., Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–1158. - PMC - PubMed
-
- Eyck B.M., van Lanschot J.J.B., Hulshof M., van der Wilk B.J., Shapiro J., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L., van Laarhoven H.W.M., Nieuwenhuijzen G.A.P., Hospers G.A.P., Bonenkamp J.J., Cuesta M.A., Blaisse R.J.B., Busch O.R., Creemers G.M., Punt C.J.A., Plukker J.T.M., Verheul H.M.W., Spillenaar Bilgen E.J., van der Sangen M.J.C., Rozema T., Ten Kate F.J.W., Beukema J.C., Piet A.H.M., van Rij C.M., Reinders J.G., Tilanus H.W., Steyerberg E.W., van der Gaast A., Group C.S. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J. Clin. Oncol. 2021;39:1995–2004. - PubMed
-
- van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P., Richel D.J., Nieuwenhuijzen G.A., Hospers G.A., Bonenkamp J.J., Cuesta M.A., Blaisse R.J., Busch O.R., ten Kate F.J., Creemers G.J., Punt C.J., Plukker J.T., Verheul H.M., Spillenaar Bilgen E.J., van Dekken H., van der Sangen M.J., Rozema T., Biermann K., Beukema J.C., Piet A.H., van Rij C.M., Reinders J.G., Tilanus H.W., van der Gaast A., Group C. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

