A molecular device for the redox quality control of GroEL/ES substrates
- PMID: 36764293
- PMCID: PMC10044410
- DOI: 10.1016/j.cell.2023.01.013
A molecular device for the redox quality control of GroEL/ES substrates
Abstract
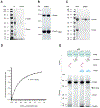
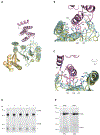
Hsp60 chaperonins and their Hsp10 cofactors assist protein folding in all living cells, constituting the paradigmatic example of molecular chaperones. Despite extensive investigations of their structure and mechanism, crucial questions regarding how these chaperonins promote folding remain unsolved. Here, we report that the bacterial Hsp60 chaperonin GroEL forms a stable, functionally relevant complex with the chaperedoxin CnoX, a protein combining a chaperone and a redox function. Binding of GroES (Hsp10 cofactor) to GroEL induces CnoX release. Cryoelectron microscopy provided crucial structural information on the GroEL-CnoX complex, showing that CnoX binds GroEL outside the substrate-binding site via a highly conserved C-terminal α-helix. Furthermore, we identified complexes in which CnoX, bound to GroEL, forms mixed disulfides with GroEL substrates, indicating that CnoX likely functions as a redox quality-control plugin for GroEL. Proteins sharing structural features with CnoX exist in eukaryotes, suggesting that Hsp60 molecular plugins have been conserved through evolution.
Keywords: TPR; chaperones; chaperonin; protein folding; proteostasis; redox; thioredoxin.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Anfinsen CB (1973). Principles that govern the folding of protein chains. Science 181, 223–230. - PubMed
-
- Jahn TR, and Radford SE (2005). The Yin and Yang of protein folding. The FEBS journal 272, 5962–5970. - PubMed
-
- Ellis RJ (2001). Macromolecular crowding: obvious but underappreciated. Trends in biochemical sciences 26, 597–604. - PubMed
-
- Goloubinoff P, Christeller JT, Gatenby AA, and Lorimer GH (1989). Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfolded state depends on two chaperonin proteins and Mg-ATP. Nature 342, 884–889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

