Update on Hepatobiliary Plasticity
- PMID: 36764306
- PMCID: PMC10005859
- DOI: 10.1055/s-0042-1760306
Update on Hepatobiliary Plasticity
Abstract
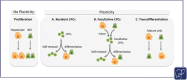
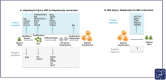
The liver field has been debating for decades the contribution of the plasticity of the two epithelial compartments in the liver, hepatocytes and biliary epithelial cells (BECs), to derive each other as a repair mechanism. The hepatobiliary plasticity has been first observed in diseased human livers by the presence of biphenotypic cells expressing hepatocyte and BEC markers within bile ducts and regenerative nodules or budding from strings of proliferative BECs in septa. These observations are not surprising as hepatocytes and BECs derive from a common fetal progenitor, the hepatoblast, and, as such, they are expected to compensate for each other's loss in adults. To investigate the cell origin of regenerated cell compartments and associated molecular mechanisms, numerous murine and zebrafish models with ability to trace cell fates have been extensively developed. This short review summarizes the clinical and preclinical studies illustrating the hepatobiliary plasticity and its potential therapeutic application.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
None declared.
Figures
References
-
- Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14(05):561–574. - PubMed
-
- Kamiya A, Kakinuma S, Yamazaki Y, Nakauchi H.Enrichment and clonal culture of progenitor cells during mouse postnatal liver development in mice Gastroenterology 2009137031114–1126., 1126.e1–1126.e14 - PubMed
-
- Okabe M, Tsukahara Y, Tanaka M. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136(11):1951–1960. - PubMed

