A multivalent polyomavirus vaccine elicits durable neutralizing antibody responses in macaques
- PMID: 36764908
- PMCID: PMC9992340
- DOI: 10.1016/j.vaccine.2023.02.002
A multivalent polyomavirus vaccine elicits durable neutralizing antibody responses in macaques
Abstract
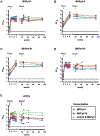
In 2019, there were about 100,000 kidney transplants globally, with more than a quarter of them performed in the United States. Unfortunately, some engrafted organs are lost to polyomavirus-associated nephropathy (PyVAN) caused by BK and JC viruses (BKPyV and JCPyV). Both viruses cause brain disease and possibly bladder cancer in immunosuppressed individuals. Transplant patients are routinely monitored for BKPyV viremia, which is an accepted hallmark of nascent nephropathy. If viremia is detected, a reduction in immunosuppressive therapy is standard care, but the intervention comes with increased risk of immune rejection of the engrafted organ. Recent reports have suggested that transplant recipients with high levels of polyomavirus-neutralizing antibodies are protected against PyVAN. Virus-like particle (VLP) vaccines, similar to approved human papillomavirus vaccines, have an excellent safety record and are known to induce high levels of neutralizing antibodies and long-lasting protection from infection. In this study, we demonstrate that VLPs representing BKPyV genotypes I, II, and IV, as well as JCPyV genotype 2 produced in insect cells elicit robust antibody titers. In rhesus macaques, all monkeys developed neutralizing antibody titers above a previously proposed protective threshold of 10,000. A second inoculation, administered 19 weeks after priming, boosted titers to a plateau of ≥ 25,000 that was maintained for almost two years. No vaccine-related adverse events were observed in any macaques. A multivalent BK/JC VLP immunogen did not show inferiority compared to the single-genotype VLP immunogens. Considering these encouraging results, we believe a clinical trial administering the multivalent VLP vaccine in patients waiting to receive a kidney transplant is warranted to evaluate its ability to reduce or eliminate PyVAN.
Keywords: BKV; HPyV1; HPyV2; JCV; PyVAN; Transplant.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Christopher B. Buck and Dr. Diana V. Pastrana are both inventors in the following: Patent # 9764022 Methods and compositions for inhibiting polyomavirus-associated pathology; and patent # 9931393 Immunogenic JC polyomavirus compositions and methods for use.
Figures


References
-
- Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87(7):1019–26. - PubMed
-
- Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277–86. - PubMed
-
- Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347(7):488–96. - PubMed
-
- Nickeleit V, Hirsch HH, Zeiler M, Gudat F, Prince O, Thiel G, et al. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15(3):324–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

