Assessing overdiagnosis of fecal immunological test screening for colorectal cancer with a digital twin approach
- PMID: 36765093
- PMCID: PMC9918445
- DOI: 10.1038/s41746-023-00763-5
Assessing overdiagnosis of fecal immunological test screening for colorectal cancer with a digital twin approach
Abstract
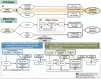
Evaluating the magnitude of overdiagnosis associated with stool-based service screening for colorectal cancer (CRC) beyond a randomized controlled trial is often intractable and understudied. We aim to estimate the proportion of overdiagnosis in population-based service screening programs for CRC with the fecal immunochemical test (FIT). The natural process of overdiagnosis-embedded disease was first built up to learn transition parameters that quantify the pathway of non-progressive and progressive screen-detected cases calibrated with sensitivity, while also taking competing mortality into account. The Markov algorithms were then developed for estimating these transition parameters based on Taiwan FIT service CRC screening data on 5,417,699 residents aged 50-69 years from 2004 to 2014. Following the digital twin design with the parallel universe structure for emulating the randomized controlled trial, the screened twin, mirroring the control group without screening, was virtually recreated by the application of the above-mentioned trained parameters to predict CRC cases containing overdiagnosis. The ratio of the predicted CRCs derived from the screened twin to the observed CRCs of the control group minus 1 was imputed to measure the extent of overdiagnosis. The extent of overdiagnosis for invasive CRCs resulting from FIT screening is 4.16% (95% CI: 2.61-5.78%). The corresponding figure is increased to 9.90% (95% CI: 8.41-11.42%) for including high grade dysplasia (HGD) and further inflated to 15.83% (95% CI: 15.23-16.46%) when the removal adenoma is considered. The modest proportion of overdiagnosis modelled by the digital twin method, dispensing with the randomized controlled trial design, suggests the harm done to population-based FIT service screening is negligible.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Fecal occult blood test for colorectal cancer screening: an evidence-based analysis.Ont Health Technol Assess Ser. 2009;9(10):1-40. Epub 2009 Sep 1. Ont Health Technol Assess Ser. 2009. PMID: 23074514 Free PMC article.
-
Analysis of the effectiveness of two noninvasive fecal tests used to screen for colorectal cancer in average-risk adults.Public Health. 2020 May;182:70-76. doi: 10.1016/j.puhe.2020.01.021. Epub 2020 Mar 13. Public Health. 2020. PMID: 32179290
-
Interval Colorectal Cancer Incidence Among Subjects Undergoing Multiple Rounds of Fecal Immunochemical Testing.Gastroenterology. 2017 Aug;153(2):439-447.e2. doi: 10.1053/j.gastro.2017.05.004. Epub 2017 May 5. Gastroenterology. 2017. PMID: 28483499
-
Diagnostic performance of flexible sigmoidoscopy combined with fecal immunochemical test in colorectal cancer screening: meta-analysis and modeling.Eur J Epidemiol. 2017 Jun;32(6):481-493. doi: 10.1007/s10654-017-0279-2. Epub 2017 Jun 30. Eur J Epidemiol. 2017. PMID: 28667446 Review.
-
Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: a systematic review and meta-analysis.Gut. 2019 May;68(5):873-881. doi: 10.1136/gutjnl-2017-315340. Epub 2018 Jun 22. Gut. 2019. PMID: 29934436
Cited by
-
Advancing Precision Oncology with Digital and Virtual Twins: A Scoping Review.Cancers (Basel). 2024 Nov 13;16(22):3817. doi: 10.3390/cancers16223817. Cancers (Basel). 2024. PMID: 39594772 Free PMC article.
-
Definitions and Characteristics of Patient Digital Twins Being Developed for Clinical Use: Scoping Review.J Med Internet Res. 2024 Nov 13;26:e58504. doi: 10.2196/58504. J Med Internet Res. 2024. PMID: 39536311 Free PMC article.
-
Evolution of digital twins in precision health applications: a scoping review study.Res Sq [Preprint]. 2024 Aug 7:rs.3.rs-4612942. doi: 10.21203/rs.3.rs-4612942/v1. Res Sq. 2024. PMID: 39149471 Free PMC article. Preprint.
-
Balancing early detection and over-screening: Evaluating colonoscopy's role in shaping colorectal cancer trends in Korea.World J Gastrointest Oncol. 2025 Mar 15;17(3):102858. doi: 10.4251/wjgo.v17.i3.102858. World J Gastrointest Oncol. 2025. PMID: 40092921 Free PMC article.
-
Digital twins for chronic lung diseases.Eur Respir Rev. 2024 Dec 18;33(174):240159. doi: 10.1183/16000617.0159-2024. Print 2024 Oct. Eur Respir Rev. 2024. PMID: 39694590 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

