Spiders' digestive system as a source of trypsin inhibitors: functional activity of a member of atracotoxin structural family
- PMID: 36765114
- PMCID: PMC9918498
- DOI: 10.1038/s41598-023-29576-y
Spiders' digestive system as a source of trypsin inhibitors: functional activity of a member of atracotoxin structural family
Abstract
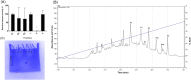
Spiders are important predators of insects and their venoms play an essential role in prey capture. Spider venoms have several potential applications as pharmaceutical compounds and insecticides. However, transcriptomic and proteomic analyses of the digestive system (DS) of spiders show that DS is also a rich source of new peptidase inhibitor molecules. Biochemical, transcriptomic and proteomic data of crude DS extracts show the presence of molecules with peptidase inhibitor potential in the spider Nephilingis cruentata. Therefore, the aims of this work were to isolate and characterize molecules with trypsin inhibitory activity. The DS of fasting adult females was homogenized under acidic conditions and subjected to heat treatment. After that, samples were submitted to ion exchange batch and high-performance reverse-phase chromatography. The fractions with trypsin inhibitory activity were confirmed by mass spectrometry, identifying six molecules with inhibitory potential. The inhibitor NcTI (Nephilingis cruentata trypsin inhibitor) was kinetically characterized, showing a KD value of 30.25 nM ± 8.13. Analysis of the tertiary structure by molecular modeling using Alpha-Fold2 indicates that the inhibitor NcTI structurally belongs to the MIT1-like atracotoxin family. This is the first time that a serine peptidase inhibitory function is attributed to this structural family and the inhibitor reactive site residue is identified. Sequence analysis indicates that these molecules may be present in the DS of other spiders and could be associated to the inactivation of prey trypsin (serine peptidase) ingested by the spiders.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




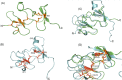

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

