Context-specific regulation of extracellular vesicle biogenesis and cargo selection
- PMID: 36765164
- PMCID: PMC10330318
- DOI: 10.1038/s41580-023-00576-0
Context-specific regulation of extracellular vesicle biogenesis and cargo selection
Abstract
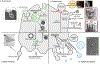
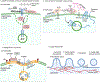
To coordinate, adapt and respond to biological signals, cells convey specific messages to other cells. An important aspect of cell-cell communication involves secretion of molecules into the extracellular space. How these molecules are selected for secretion has been a fundamental question in the membrane trafficking field for decades. Recently, extracellular vesicles (EVs) have been recognized as key players in intercellular communication, carrying not only membrane proteins and lipids but also RNAs, cytosolic proteins and other signalling molecules to recipient cells. To communicate the right message, it is essential to sort cargoes into EVs in a regulated and context-specific manner. In recent years, a wealth of lipidomic, proteomic and RNA sequencing studies have revealed that EV cargo composition differs depending upon the donor cell type, metabolic cues and disease states. Analyses of distinct cargo 'fingerprints' have uncovered mechanistic linkages between the activation of specific molecular pathways and cargo sorting. In addition, cell biology studies are beginning to reveal novel biogenesis mechanisms regulated by cellular context. Here, we review context-specific mechanisms of EV biogenesis and cargo sorting, focusing on how cell signalling and cell state influence which cellular components are ultimately targeted to EVs.
© 2023. Springer Nature Limited.
Conflict of interest statement
Declaration of Interests: There are no declarations of interests.
Figures

References
-
- Harding C, Heuser J & Stahl P Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 35, 256–263 (1984). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

