Time-varying SUVr reflects the dynamics of dopamine increases during methylphenidate challenges in humans
- PMID: 36765261
- PMCID: PMC9918528
- DOI: 10.1038/s42003-023-04545-3
Time-varying SUVr reflects the dynamics of dopamine increases during methylphenidate challenges in humans
Abstract
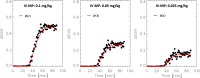
Dopamine facilitates cognition and is implicated in reward processing. Methylphenidate, a dopamine transporter blocker widely used to treat attention-deficit/hyperactivity disorder, can have rewarding and addictive effects if injected. Since methylphenidate's brain uptake is much faster after intravenous than oral intake, we hypothesize that the speed of dopamine increases in the striatum in addition to its amplitude underly drug reward. To test this we use simulations and PET data of [11C]raclopride's binding displacement with oral and intravenous methylphenidate challenges in 20 healthy controls. Simulations suggest that the time-varying difference in standardized uptake value ratios for [11C]raclopride between placebo and methylphenidate conditions is a proxy for the time-varying dopamine increases induced by methylphenidate. Here we show that the dopamine increase induced by intravenous methylphenidate (0.25 mg/kg) in the striatum is significantly faster than that by oral methylphenidate (60 mg), and its time-to-peak is strongly associated with the intensity of the self-report of "high". We show for the first time that the "high" is associated with the fast dopamine increases induced by methylphenidate.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Volkow N, Fowler J, Wang G, Ding Y, Gatley S. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur. Neuropsychopharmaco. 2002;12:557–566. - PubMed
-
- Volkow N, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch. Gen. Psychiatry. 1995;52:456–463. - PubMed
-
- Volkow N, et al. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999;31:59–66. - PubMed
-
- Volkow N, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. - PubMed
-
- Klein-Schwartz W. Abuse and toxicity of methylphenidate. Curr. Opin. Pediatr. 2002;14:219–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

