Pristane induced lupus mice as a model for neuropsychiatric lupus (NPSLE)
- PMID: 36765366
- PMCID: PMC9921421
- DOI: 10.1186/s12993-023-00205-y
Pristane induced lupus mice as a model for neuropsychiatric lupus (NPSLE)
Abstract
Background: The pristane-induced lupus (PIL) model is a useful tool for studying environmental-related systemic lupus erythematosus (SLE). However, neuropsychiatric manifestations in this model have not been investigated in detail. Because neuropsychiatric lupus (NPSLE) is an important complication of SLE, we investigated the neuropsychiatric symptoms in the PIL mouse model to evaluate its suitability for NPSLE studies.
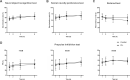
Results: PIL mice showed olfactory dysfunction accompanied by an anxiety- and depression-like phenotype at month 2 or 4 after pristane injection. The levels of cytokines (IL-1β, IFN-α, IFN-β, IL-10, IFN-γ, IL-6, TNF-α and IL-17A) and chemokines (CCL2 and CXCL10) in the brain and blood-brain barrier (BBB) permeability increased significantly from week 2 or month 1, and persisted throughout the observed course of the disease. Notably, IgG deposition in the choroid plexus and lateral ventricle wall were observed at month 1 and both astrocytes and microglia were activated. Persistent activation of astrocytes was detected throughout the observed course of the disease, while microglial activation diminished dramatically at month 4. Lipofuscin deposition, a sign of neuronal damage, was detected in cortical and hippocampal neurons from month 4 to 8.
Conclusion: PIL mice exhibit a series of characteristic behavioral deficits and pathological changes in the brain, and therefore might be suitable for investigating disease pathogenesis and for evaluating potential therapeutic targets for environmental-related NPSLE.
Keywords: Behavioral deficit; Cytokine; Glia cells; IgG; Lipofuscin; Mouse model; Neuropsychiatric lupus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Optogenetic stimulation of basal forebrain cholinergic neurons prevents neuroinflammation and neuropsychiatric manifestations in pristane induced lupus mice.Behav Brain Funct. 2023 Jun 15;19(1):11. doi: 10.1186/s12993-023-00213-y. Behav Brain Funct. 2023. PMID: 37322485 Free PMC article.
-
Lupus animal models and neuropsychiatric implications.Clin Rheumatol. 2021 Jul;40(7):2535-2545. doi: 10.1007/s10067-020-05493-7. Epub 2020 Nov 6. Clin Rheumatol. 2021. PMID: 33155159 Review.
-
Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus.Ann Rheum Dis. 2023 May;82(5):646-657. doi: 10.1136/ard-2022-223506. Epub 2023 Mar 10. Ann Rheum Dis. 2023. PMID: 36898766 Free PMC article.
-
Ectopic CD4+ T cells in choroid plexus mediate neuropsychiatric lupus symptoms in mice via interferon-γ induced microglia activation.J Autoimmun. 2024 May;145:103199. doi: 10.1016/j.jaut.2024.103199. Epub 2024 Mar 6. J Autoimmun. 2024. PMID: 38452512
-
Advances in the diagnosis, pathogenesis and treatment of neuropsychiatric systemic lupus erythematosus.Curr Opin Rheumatol. 2020 Mar;32(2):152-158. doi: 10.1097/BOR.0000000000000682. Curr Opin Rheumatol. 2020. PMID: 31895125 Free PMC article. Review.
Cited by
-
The Involvement of Glial Cells in Blood-Brain Barrier Damage in Neuroimmune Diseases.Int J Mol Sci. 2024 Nov 17;25(22):12323. doi: 10.3390/ijms252212323. Int J Mol Sci. 2024. PMID: 39596390 Free PMC article. Review.
-
What is known about the effects of vitamin D in neuropsychiatric lupus?Adv Rheumatol. 2024 Jan 2;64(1):2. doi: 10.1186/s42358-023-00344-w. Adv Rheumatol. 2024. PMID: 38167239 Review.
-
Optogenetic stimulation of basal forebrain cholinergic neurons prevents neuroinflammation and neuropsychiatric manifestations in pristane induced lupus mice.Behav Brain Funct. 2023 Jun 15;19(1):11. doi: 10.1186/s12993-023-00213-y. Behav Brain Funct. 2023. PMID: 37322485 Free PMC article.
-
SVM-Based Model Combining Patients' Reported Outcomes and Lymphocyte Phenotypes of Depression in Systemic Lupus Erythematosus.Biomolecules. 2023 Apr 23;13(5):723. doi: 10.3390/biom13050723. Biomolecules. 2023. PMID: 37238593 Free PMC article.
-
Distinct ACC Neural Mechanisms Underlie Authentic and Transmitted Anxiety Induced by Maternal Separation in Mice.J Neurosci. 2023 Nov 29;43(48):8201-8218. doi: 10.1523/JNEUROSCI.0558-23.2023. J Neurosci. 2023. PMID: 37845036 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2021M703606/the fellowship of China Postdoctoral Science Foundation
- 2020JH2/10100014/Department of Science and Technology of Liaoning Province
- XLYC2002094/"Xingliao Talent Plan" of Liaoning, China
- XLYC2002062/"Xingliao Talent Plan" of Liaoning, China
- 19-109-4-15/the Project for Construction of Key Platform, Shenyang, China
LinkOut - more resources
Full Text Sources
Medical

