Multi-Parameter Analysis of Disseminated Tumor Cells (DTCs) in Early Breast Cancer Patients with Hormone-Receptor-Positive Tumors
- PMID: 36765527
- PMCID: PMC9913363
- DOI: 10.3390/cancers15030568
Multi-Parameter Analysis of Disseminated Tumor Cells (DTCs) in Early Breast Cancer Patients with Hormone-Receptor-Positive Tumors
Abstract
Background: Patients with hormone-receptor-positive (HR+) breast cancer are at increased risk for late recurrence. One reason might be disseminated tumor cells (DTCs), which split off in the early stages of the disease and metastasize into the bone marrow (BM).
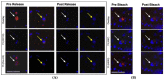
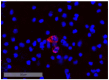
Methods: We developed a novel multi-parameter immunofluorescence staining protocol using releasable and bleachable antibody-fluorochrome-conjugates. This sequential procedure enabled us to analyze six distinct phenotypical and therapy-related markers on the same DTC. We characterized BM aspirates from 29 patients with a HR+ tumor and a known positive DTC status-based on the standardized detection of epithelial cells in BM.
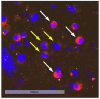
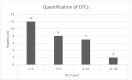
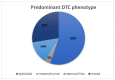
Results: Using the immunofluorescence staining, a total of 153 DTCs were detected. Luminal A patients revealed a higher DTC count compared with luminal B. The majority of the detected DTCs were CK-positive (128/153). However, in 16 of 17 luminal A patients we found HER2-positive DTCs. We detected CK-negative DTCs (25/153) in 12 of 29 patients. Of those cells, 76% were Ki67-positive and 68% were HER2-positive. Moreover, we detected DTC clusters consisting of mixed characteristics in 6 of 29 patients.
Conclusions: Using sequential multi-parameter imaging made it possible to identify distinct DTC profiles not solely based on epithelial features. Our findings indicate that characterization rather than quantification of DTCs might be relevant for treatment decisions.
Keywords: HER2; bone marrow; breast cancer; disseminated tumor cells; dormancy; hormone receptor; proliferation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ramamoorthi G., Kodumudi K., Gallen C., Zachariah N.N., Basu A., Albert G., Beyer A., Snyder C., Wiener D., Costa R.L.B., et al. Disseminated cancer cells in breast cancer: Mechanism of dissemination and dormancy and emerging insights on therapeutic opportunities. Semin. Cancer Biol. 2022;78:78–89. doi: 10.1016/j.semcancer.2021.02.004. - DOI - PubMed
-
- Pantel K., Schlimok G., Braun S., Kutter D., Lindemann F., Schaller G., Funke I., Izbicki J.R., Riethmüller G. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl. Cancer Inst. 1993;85:1419–1424. doi: 10.1093/jnci/85.17.1419. - DOI - PubMed
-
- Janni W., Vogl F.D., Wiedswang G., Synnestvedt M., Fehm T., Jückstock J., Borgen E., Rack B., Braun S., Sommer H., et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17:2967–2976. doi: 10.1158/1078-0432.CCR-10-2515. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

