Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells
- PMID: 36765558
- PMCID: PMC9913141
- DOI: 10.3390/cancers15030601
Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells
Abstract
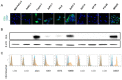
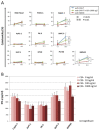
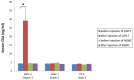
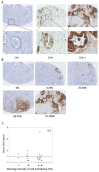
Chimeric antigen receptor engineered T cell (CAR-T) therapy has high therapeutic efficacy against blood cancers, but it has not shown satisfactory results in solid tumors. Therefore, we examined the therapeutic effect of CAR-T therapy targeting carcinoembryonic antigen (CEA) in pancreatic adenocarcinoma (PDAC). CEA expression levels on the cell membranes of various PDAC cell lines were evaluated using flow cytometry and the cells were divided into high, medium, and low expression groups. The relationship between CEA expression level and the antitumor effect of anti-CEA-CAR-T was evaluated using a functional assay for various PDAC cell lines; a significant correlation was observed between CEA expression level and the antitumor effect. We created orthotopic PDAC xenograft mouse models and injected with anti-CEA-CAR-T; only the cell line with high CEA expression exhibited a significant therapeutic effect. Thus, the therapeutic effect of CAR-T therapy was related to the target antigen expression level, and the further retrospective analysis of pathological findings from PDAC patients showed a correlation between the intensity of CEA immunostaining and tumor heterogeneity. Therefore, CEA expression levels in biopsies or surgical specimens can be clinically used as biomarkers to select PDAC patients for anti-CAR-T therapy.
Keywords: adoptive cell therapy; carcinoembryonic antigen; chimeric antigen receptor engineered T cell; orthotopic xenograft mouse model; pancreatic ductal carcinoma.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Figures




References
-
- Cielito C.R., Wenyaw C., James L.A., Henry Q.X., Linus H., Douglas B.E., Gauri V., Samrat B., Robert A.W., Christopher C. Patterns of self-reported symptoms in pancreatic cancer patients receiving chemoradiation. J. Pain Symptom. Manag. 2007;34:244–252. doi: 10.1016/j.jpainsymman.2006.11.007. - DOI - PMC - PubMed
-
- Kawalekar O.U., O’ Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey Jr A.D., Patel P.R., Guedan S., Scholler J., Keith B., et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:712. doi: 10.1016/j.immuni.2016.02.023. - DOI - PubMed
LinkOut - more resources
Full Text Sources

