The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study
- PMID: 36765591
- PMCID: PMC9913459
- DOI: 10.3390/cancers15030633
The Use of a Non-Invasive Biomarker for Colorectal Cancer Screening: A Comparative Cost-Effectiveness Modeling Study
Abstract
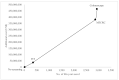
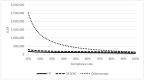
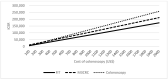
This study aimed to examine the cost-effectiveness of fecal biomarker M3 panel compared to fecal immunochemical test (FIT) and colonoscopy in an Asian population. In a hypothetical population of 100,000 persons aged 50 years who received FIT yearly, M3 biomarker yearly, or colonoscopy every 10 years until the age of 75 years. Participants with positive FOBT or a result of "high risk" identified using the M3 biomarker are offered colonoscopy. We assumed surveillance colonoscopy is repeated every 3 years, and examined the treatment cost. A comparison of various outcome measures was conducted using Markov modelling. The incremental cost-effectiveness ratio (ICER) of FIT, M3 biomarker, and colonoscopy was USD108,176, USD133,485 and USD159,596, respectively. Comparing with FIT, the use of M3 biomarker could lead to significantly smaller total loss of cancer-related life-years (2783 vs. 5279); a higher number of CRC cases prevented (1622 vs. 146), a higher proportion of CRC cases prevented (50.2% vs. 4.5%), more life-years saved (2852 vs. 339), and cheaper total costs per life-year saved (USD212,553 vs. 773,894). The total costs per life-year saved is more affordable than that achieved by colonoscopy as a primary screening tool (USD212,553 vs. USD236,909). The findings show that M3 biomarkers may be more cost-effective than colonoscopy.
Keywords: colorectal cancer; cost-effectiveness; fecal immunochemical tests; non-invasive biomarker; screening.
Conflict of interest statement
The fecal biomarker M3 used in this paper is a product of GenieBiome limited. M.C.S.W. has been serving as an advisory committee member for Pfizer, and an external expert for GlaxoSmithKline Limited. He has been paid consultancy fees for providing advice on research. He is an Honorary Advisor of GenieBiome limited. S.C.N. has served as speakers for Janssen, Abbvie, Takeda, Ferring, Tilotts, Menarini, Pfizer and have received research grants from Olympus, Ferring, Janssen and Abbvie. She is scientific cofounder of GenieBiome limited. F.K.L.C. has served as a consultant to Eisai, Pfizer, Takeda, and Otsuka, and has been paid lecture fees by Eisai, Pfizer, AstraZeneca, and Takeda. The other authors declare that they have no competing interests.
Figures

Similar articles
-
Health benefits and cost-effectiveness of a hybrid screening strategy for colorectal cancer.Clin Gastroenterol Hepatol. 2013 Sep;11(9):1158-66. doi: 10.1016/j.cgh.2013.03.013. Epub 2013 Mar 28. Clin Gastroenterol Hepatol. 2013. PMID: 23542330
-
Cost-effectiveness and budget impact analyses of colorectal cancer screenings in a low- and middle-income country: example from Thailand.J Med Econ. 2019 Dec;22(12):1351-1361. doi: 10.1080/13696998.2019.1674065. Epub 2019 Oct 12. J Med Econ. 2019. PMID: 31560247
-
Cost-effectiveness of screening for colorectal cancer in the general population.JAMA. 2000 Oct 18;284(15):1954-61. doi: 10.1001/jama.284.15.1954. JAMA. 2000. PMID: 11035892
-
Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: a systematic review and meta-analysis.Gastrointest Endosc. 2020 Mar;91(3):684-697.e15. doi: 10.1016/j.gie.2019.11.035. Epub 2019 Nov 30. Gastrointest Endosc. 2020. PMID: 31790657
-
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer.Am J Gastroenterol. 2017 Jul;112(7):1016-1030. doi: 10.1038/ajg.2017.174. Epub 2017 Jun 6. Am J Gastroenterol. 2017. PMID: 28555630 Review.
Cited by
-
Advances in extracellular vesicle (EV) biomarkers for precision diagnosis and therapeutic in colorectal cancer.Front Oncol. 2025 Jul 16;15:1581015. doi: 10.3389/fonc.2025.1581015. eCollection 2025. Front Oncol. 2025. PMID: 40740862 Free PMC article. Review.
-
From Crypts to Cancer: A Holistic Perspective on Colorectal Carcinogenesis and Therapeutic Strategies.Int J Mol Sci. 2024 Aug 30;25(17):9463. doi: 10.3390/ijms25179463. Int J Mol Sci. 2024. PMID: 39273409 Free PMC article. Review.
-
Balancing early detection and over-screening: Evaluating colonoscopy's role in shaping colorectal cancer trends in Korea.World J Gastrointest Oncol. 2025 Mar 15;17(3):102858. doi: 10.4251/wjgo.v17.i3.102858. World J Gastrointest Oncol. 2025. PMID: 40092921 Free PMC article.
References
LinkOut - more resources
Full Text Sources

