Clear Cell Renal Cell Carcinoma: From Biology to Treatment
- PMID: 36765622
- PMCID: PMC9913203
- DOI: 10.3390/cancers15030665
Clear Cell Renal Cell Carcinoma: From Biology to Treatment
Abstract
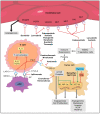
The majority of kidney cancers are detected incidentally and typically diagnosed at a localized stage, however, the development of regional or distant disease occurs in one-third of patients. Over 90% of kidney tumors are renal cell carcinomas, of which, clear cell is the most predominate histologic subtype. Von Hippel Lindau (VHL) gene alterations result in the overexpression of growth factors that are central to the pathogenesis of clear cell carcinoma. The therapeutic strategies have revolved around this tumor suppressor gene and have led to the approval of tyrosine kinase inhibitors (TKI) targeting the vascular endothelial growth factor (VEGF) axis. The treatment paradigm shifted with the introduction of immune checkpoint inhibitors (ICI) and programed death-1 (PD-1) inhibition, leading to durable response rates and improved survival. Combinations of TKI and/or ICIs have become the standard of care for advanced clear cell renal cell carcinoma (ccRCC), changing the outlook for patients, with several new and promising therapeutic targets under development.
Keywords: VEGF; clear cell renal cell carcinoma; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Klapper J.A., Downey S.G., Smith F.O., Yang J.C., Hughes M.S., Kammula U.S., Sherry R.M., Royal R.E., Steinberg S.M., Rosenberg S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources