Targeting the Hedgehog Pathway in Rhabdomyosarcoma
- PMID: 36765685
- PMCID: PMC9913695
- DOI: 10.3390/cancers15030727
Targeting the Hedgehog Pathway in Rhabdomyosarcoma
Abstract
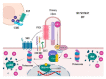
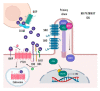
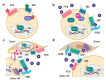
Aberrant activation of the Hedgehog (Hh) signalling pathway is known to play an oncogenic role in a wide range of cancers; in the particular case of rhabdomyosarcoma, this pathway has been demonstrated to be an important player for both oncogenesis and cancer progression. In this review, after a brief description of the pathway and the characteristics of its molecular components, we describe, in detail, the main activation mechanisms that have been found in cancer, including ligand-dependent, ligand-independent and non-canonical activation. In this context, the most studied inhibitors, i.e., SMO inhibitors, have shown encouraging results for the treatment of basal cell carcinoma and medulloblastoma, both tumour types often associated with mutations that lead to the activation of the pathway. Conversely, SMO inhibitors have not fulfilled expectations in tumours-among them sarcomas-mostly associated with ligand-dependent Hh pathway activation. Despite the controversy existing regarding the results obtained with SMO inhibitors in these types of tumours, several compounds have been (or are currently being) evaluated in sarcoma patients. Finally, we discuss some of the reasons that could explain why, in some cases, encouraging preclinical data turned into disappointing results in the clinical setting.
Keywords: BOC; CDO; Desert; GAS1; Hh pathway; Indian; PTCH; SMO; STS; SUFU; Sonic; cancer; embryonic pathways; paediatric cancer; soft tissue sarcomas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hibbitts E., Chi Y.Y., Hawkins D.S., Barr F.G., Bradley J.A., Dasgupta R., Meyer W.H., Rodeberg D.A., Rudzinski E.R., Spunt S.L., et al. Refinement of Risk Stratification for Childhood Rhabdomyosarcoma Using FOXO1 Fusion Status in Addition to Established Clinical Outcome Predictors: A Report from the Children’s Oncology Group. Cancer Med. 2019;8:6437–6448. doi: 10.1002/cam4.2504. - DOI - PMC - PubMed
-
- Gallego S., Zanetti I., Orbach D., Ranchère D., Shipley J., Zin A., Bergeron C., de Salvo G.L., Chisholm J., Ferrari A., et al. Fusion Status in Patients with Lymph Node-Positive (N1) Alveolar Rhabdomyosarcoma Is a Powerful Predictor of Prognosis: Experience of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG) Cancer. 2018;124:3201–3209. doi: 10.1002/cncr.31553. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

