Sialyl LewisX/A and Cytokeratin Crosstalk in Triple Negative Breast Cancer
- PMID: 36765690
- PMCID: PMC9913872
- DOI: 10.3390/cancers15030731
Sialyl LewisX/A and Cytokeratin Crosstalk in Triple Negative Breast Cancer
Abstract
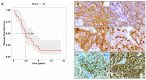
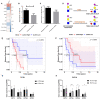
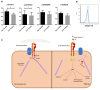
Triple-negative breast cancer (TNBC) encompasses multiple entities and is generally highly aggressive and metastatic. We aimed to determine the clinical and biological relevance of Sialyl-Lewis X and A (sLeX/A)-a fucosylated glycan involved in metastasis-in TNBC. Here, we studied tissues from 50 TNBC patients, transcripts from a TNBC dataset from The Cancer Genome Atlas (TCGA) database, and a primary breast cancer cell line. All 50 TNBC tissue samples analysed expressed sLeX/A. Patients with high expression of sLeX/A had 3 years less disease-free survival than patients with lower expression. In tissue, sLeX/A negatively correlated with cytokeratins 5/6 (CK5/6, which was corroborated by the inverse correlation between fucosyltransferases and CK5/6 genes. Our observations were confirmed in vitro when inhibition of sLeX/A remarkably increased expression of CK5/6, followed by a decreased proliferation and invasion capacity. Among the reported glycoproteins bearing sLeX/A and based on the STRING tool, α6 integrin showed the highest interaction score with CK5/6. This is the first report on the sLeX/A expression in TNBC, highlighting its association with lower disease-free survival and its inverse crosstalk with CK5/6 with α6 integrin as a mediator. All in all, sLeX/A is critical for TNBC malignancy and a potential prognosis biomarker and therapeutic target.
Keywords: aberrant glycosylation; cytokeratin expression; disease-free survival rate; intermediate filament proteins; sialyl LewisX/A (sLeX/A); triple-negative breast cancer (TNBC); α6 integrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E., Zackrisson S., Cardoso F. ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015;26((Suppl. 5)):v8–v30. doi: 10.1093/annonc/mdv298. - DOI - PubMed
-
- Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., Lickley L.A., Rawlinson E., Sun P., Narod S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

