Potent Inhibition of Macropinocytosis by Niclosamide in Cancer Cells: A Novel Mechanism for the Anticancer Efficacy for the Antihelminthic
- PMID: 36765717
- PMCID: PMC9913174
- DOI: 10.3390/cancers15030759
Potent Inhibition of Macropinocytosis by Niclosamide in Cancer Cells: A Novel Mechanism for the Anticancer Efficacy for the Antihelminthic
Abstract
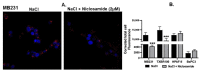
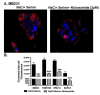
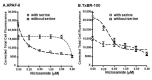
Niclosamide, a drug used to treat tapeworm infection, possesses anticancer effects by interfering with multiple signaling pathways. Niclosamide also causes intracellular acidification. We have recently discovered that the amino acid transporter SLC38A5, an amino acid-dependent Na+/H+ exchanger, activates macropinocytosis in cancer cells via amino acid-induced intracellular alkalinization. Therefore, we asked whether niclosamide will block basal and SLC38A5-mediated macropinocytosis via intracellular acidification. We monitored macropinocytosis in pancreatic and breast cancer cells using TMR-dextran and the function of SLC38A5 by measuring Li+-stimulated serine uptake. The peptide transporter activity was measured by the uptake of glycylsarcosine. Treatment of the cancer cells with niclosamide caused intracellular acidification. The drug blocked basal and serine-induced macropinocytosis with differential potency, with an EC50 of ~5 μM for the former and ~0.4 μM for the latter. The increased potency for amino acid-mediated macropinocytosis is due to direct inhibition of SLC38A5 by niclosamide in addition to the ability of the drug to cause intracellular acidification. The drug also inhibited the activity of the H+-coupled peptide transporter. We conclude that niclosamide induces nutrient starvation in cancer cells by blocking macropinocytosis, SLC38A5 and the peptide transporter. These studies uncover novel, hitherto unknown, mechanisms for the anticancer efficacy of this antihelminthic.
Keywords: Na+/H+ exchanger; SLC38A5; amino acid transporter; amino acid-mediated Na+/H+ exchange; antihelminthic; intracellular acidification; macropinocytosis; niclosamide; peptide transport.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






Similar articles
-
Impact of Oncogenic Changes in p53 and KRAS on Macropinocytosis and Ferroptosis in Colon Cancer Cells and Anticancer Efficacy of Niclosamide with Differential Effects on These Two Processes.Cells. 2024 May 30;13(11):951. doi: 10.3390/cells13110951. Cells. 2024. PMID: 38891084 Free PMC article.
-
Induction of Oxidative Stress and Ferroptosis in Triple-Negative Breast Cancer Cells by Niclosamide via Blockade of the Function and Expression of SLC38A5 and SLC7A11.Antioxidants (Basel). 2024 Feb 27;13(3):291. doi: 10.3390/antiox13030291. Antioxidants (Basel). 2024. PMID: 38539825 Free PMC article.
-
Unconventional Functions of Amino Acid Transporters: Role in Macropinocytosis (SLC38A5/SLC38A3) and Diet-Induced Obesity/Metabolic Syndrome (SLC6A19/SLC6A14/SLC6A6).Biomolecules. 2022 Jan 31;12(2):235. doi: 10.3390/biom12020235. Biomolecules. 2022. PMID: 35204736 Free PMC article. Review.
-
Niclosamide, an old antihelminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells.Chin J Cancer. 2012 Apr;31(4):178-84. doi: 10.5732/cjc.011.10290. Epub 2012 Jan 9. Chin J Cancer. 2012. PMID: 22237038 Free PMC article. Review.
-
Amino acid transporter SLC38A5 is a tumor promoter and a novel therapeutic target for pancreatic cancer.Sci Rep. 2023 Oct 6;13(1):16863. doi: 10.1038/s41598-023-43983-1. Sci Rep. 2023. PMID: 37803043 Free PMC article.
Cited by
-
Clinical safety and pharmacokinetics of a novel oral niclosamide formulation compared with marketed niclosamide chewing tablets in healthy volunteers: A three-part randomized, double-blind, placebo-controlled trial.PLoS One. 2025 Feb 25;20(2):e0303924. doi: 10.1371/journal.pone.0303924. eCollection 2025. PLoS One. 2025. PMID: 39999124 Free PMC article. Clinical Trial.
-
Amino acid metabolism in breast cancer: pathogenic drivers and therapeutic opportunities.Protein Cell. 2025 Jul 19;16(7):506-531. doi: 10.1093/procel/pwaf011. Protein Cell. 2025. PMID: 39973065 Free PMC article. Review.
-
Impact of Oncogenic Changes in p53 and KRAS on Macropinocytosis and Ferroptosis in Colon Cancer Cells and Anticancer Efficacy of Niclosamide with Differential Effects on These Two Processes.Cells. 2024 May 30;13(11):951. doi: 10.3390/cells13110951. Cells. 2024. PMID: 38891084 Free PMC article.
-
SLC38A5 promotes glutamine metabolism and inhibits cisplatin chemosensitivity in breast cancer.Breast Cancer. 2024 Jan;31(1):96-104. doi: 10.1007/s12282-023-01516-8. Epub 2023 Nov 2. Breast Cancer. 2024. PMID: 37914960
-
Induction of Oxidative Stress and Ferroptosis in Triple-Negative Breast Cancer Cells by Niclosamide via Blockade of the Function and Expression of SLC38A5 and SLC7A11.Antioxidants (Basel). 2024 Feb 27;13(3):291. doi: 10.3390/antiox13030291. Antioxidants (Basel). 2024. PMID: 38539825 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

