Systemic Oncological Treatments versus Supportive Care for Patients with Advanced Hepatobiliary Cancers: An Overview of Systematic Reviews
- PMID: 36765723
- PMCID: PMC9913533
- DOI: 10.3390/cancers15030766
Systemic Oncological Treatments versus Supportive Care for Patients with Advanced Hepatobiliary Cancers: An Overview of Systematic Reviews
Abstract
Background: The trade-off between systemic oncological treatments (SOTs) and UPSC in patients with primary advanced hepatobiliary cancers (HBCs) is not clear in terms of patient-centred outcomes beyond survival. This overview aims to assess the effectiveness of SOTs (chemotherapy, immunotherapy and targeted/biological therapies) versus UPSC in advanced HBCs.
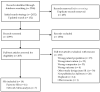
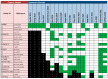
Methods: We searched for systematic reviews (SRs) in PubMed, EMBASE, the Cochrane Library, Epistemonikos and PROSPERO. Two authors assessed eligibility independently and performed data extraction. We estimated the quality of SRs and the overlap of primary studies, performed de novo meta-analyses and assessed the certainty of evidence for each outcome.
Results: We included 18 SRs, most of which were of low quality and highly overlapped. For advanced hepatocellular carcinoma, SOTs showed better overall survival (HR = 0.62, 95% CI 0.55-0.77, high certainty for first-line therapy; HR = 0.85, 95% CI 0.79-0.92, moderate certainty for second-line therapy) with higher toxicity (RR = 1.18, 95% CI 0.87-1.60, very low certainty for first-line therapy; RR = 1.58, 95% CI 1.28-1.96, low certainty for second-line therapy). Survival was also better for SOTs in advanced gallbladder cancer. No outcomes beyond survival and toxicity could be meta-analysed.
Conclusion: SOTs in advanced HBCs tend to improve survival at the expense of greater toxicity. Future research should inform other patient-important outcomes to guide clinical decision making.
Keywords: antineoplastic agents; biliary tract neoplasms; biological therapy; immunotherapy; liver neoplasms; molecular-targeted therapy; review literature as topic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

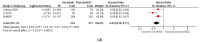
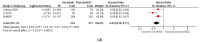

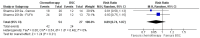
References
-
- Benson A.B., D’Angelica M.I., Abbott D.E., Anaya D.A., Anders R., Are C., Bachini M., Borad M., Brown D., Burgoyne A., et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. - DOI - PubMed
-
- Lin L., Yan L., Liu Y., Qu C., Ni J., Li H. The burden and trends of primary liver cancer caused by specific etiologies from 1990 to 2017 at the global, regional, national, age, and sex level results from the global burden of disease study 2017. Liver Cancer. 2020;9:563–582. doi: 10.1159/000508568. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

