Performance Metrics of the Scoring System for the Diagnosis of the Beckwith-Wiedemann Spectrum (BWSp) and Its Correlation with Cancer Development
- PMID: 36765732
- PMCID: PMC9913441
- DOI: 10.3390/cancers15030773
Performance Metrics of the Scoring System for the Diagnosis of the Beckwith-Wiedemann Spectrum (BWSp) and Its Correlation with Cancer Development
Abstract
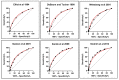
Different scoring systems for the clinical diagnosis of the Beckwith-Wiedemann spectrum (BWSp) have been developed over time, the most recent being the international consensus score. Here we try to validate and provide data on the performance metrics of these scoring systems of the 2018 international consensus and the previous ones, relating them to BWSp features, molecular tests, and the probability of cancer development in a cohort of 831 patients. The consensus scoring system had the best performance (sensitivity 0.85 and specificity 0.43). In our cohort, the diagnostic yield of tests on blood-extracted DNA was low in patients with a low consensus score (~20% with a score = 2), and the score did not correlate with cancer development. We observed hepatoblastoma (HB) in 4.3% of patients with UPD(11)pat and Wilms tumor in 1.9% of patients with isolated lateralized overgrowth (ILO). We validated the efficacy of the currently used consensus score for BWSp clinical diagnosis. Based on our observation, a first-tier analysis of tissue-extracted DNA in patients with <4 points may be considered. We discourage the use of the consensus score value as an indicator of the probability of cancer development. Moreover, we suggest considering cancer screening for negative patients with ILO (risk ~2%) and HB screening for patients with UPD(11)pat (risk ~4%).
Keywords: Beckwith–Wiedemann syndrome spectrum; genomic imprinting; score; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Weksberg R., Nishikawa J., Caluseriu O., Fei Y.L., Shuman C., Wei C., Steele L., Cameron J., Smith A., Ambus I., et al. Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum. Mol. Genet. 2001;10:2989–3000. doi: 10.1093/hmg/10.26.2989. - DOI - PubMed
-
- Maas S.M., Vansenne F., Kadouch D.J., Ibrahim A., Bliek J., Hopman S., Mannens M.M., Merks J.H., Maher E.R., Hennekam R.C. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am. J. Med. Genet. A. 2016;170:2248–2260. doi: 10.1002/ajmg.a.37801. - DOI - PubMed

