Synthesis and Significance of Arachidonic Acid, a Substrate for Cyclooxygenases, Lipoxygenases, and Cytochrome P450 Pathways in the Tumorigenesis of Glioblastoma Multiforme, Including a Pan-Cancer Comparative Analysis
- PMID: 36765904
- PMCID: PMC9913267
- DOI: 10.3390/cancers15030946
Synthesis and Significance of Arachidonic Acid, a Substrate for Cyclooxygenases, Lipoxygenases, and Cytochrome P450 Pathways in the Tumorigenesis of Glioblastoma Multiforme, Including a Pan-Cancer Comparative Analysis
Abstract
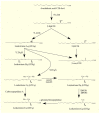
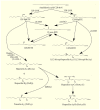
Glioblastoma multiforme (GBM) is one of the most aggressive gliomas. New and more effective therapeutic approaches are being sought based on studies of the various mechanisms of GBM tumorigenesis, including the synthesis and metabolism of arachidonic acid (ARA), an omega-6 polyunsaturated fatty acid (PUFA). PubMed, GEPIA, and the transcriptomics analysis carried out by Seifert et al. were used in writing this paper. In this paper, we discuss in detail the biosynthesis of this acid in GBM tumors, with a special focus on certain enzymes: fatty acid desaturase (FADS)1, FADS2, and elongation of long-chain fatty acids family member 5 (ELOVL5). We also discuss ARA metabolism, particularly its release from cell membrane phospholipids by phospholipase A2 (cPLA2, iPLA2, and sPLA2) and its processing by cyclooxygenases (COX-1 and COX-2), lipoxygenases (5-LOX, 12-LOX, 15-LOX-1, and 15-LOX-2), and cytochrome P450. Next, we discuss the significance of lipid mediators synthesized from ARA in GBM cancer processes, including prostaglandins (PGE2, PGD2, and 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2)), thromboxane A2 (TxA2), oxo-eicosatetraenoic acids, leukotrienes (LTB4, LTC4, LTD4, and LTE4), lipoxins, and many others. These lipid mediators can increase the proliferation of GBM cancer cells, cause angiogenesis, inhibit the anti-tumor response of the immune system, and be responsible for resistance to treatment.
Keywords: 5-HETE; 5-lipoxygenase; PUFA; arachidonic acid; cyclooxygenase-2; fatty acid; glioblastoma multiforme; leukotriene; prostaglandin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Unraveling cadmium-driven liver inflammation with a focus on arachidonic acid metabolites and TLR4/ IκBα /NF-κB pathway.Ecotoxicol Environ Saf. 2024 Nov 1;286:117177. doi: 10.1016/j.ecoenv.2024.117177. Epub 2024 Oct 16. Ecotoxicol Environ Saf. 2024. PMID: 39418721
-
Quantitative profiling of inflammatory and pro-resolving lipid mediators in human adolescents and mouse plasma using UHPLC-MS/MS.Clin Chem Lab Med. 2021 Jul 12;59(11):1811-1823. doi: 10.1515/cclm-2021-0644. Print 2021 Oct 26. Clin Chem Lab Med. 2021. PMID: 34243224
-
Role of arachidonic acid metabolites on the control of non-differentiated intestinal epithelial cell growth.Int J Biochem Cell Biol. 2013 Aug;45(8):1620-8. doi: 10.1016/j.biocel.2013.05.009. Epub 2013 May 16. Int J Biochem Cell Biol. 2013. PMID: 23685077
-
Transcellular biosynthesis of eicosanoid lipid mediators.Biochim Biophys Acta. 2015 Apr;1851(4):377-82. doi: 10.1016/j.bbalip.2014.09.002. Epub 2014 Sep 16. Biochim Biophys Acta. 2015. PMID: 25218301 Review.
-
Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets.Signal Transduct Target Ther. 2021 Feb 26;6(1):94. doi: 10.1038/s41392-020-00443-w. Signal Transduct Target Ther. 2021. PMID: 33637672 Free PMC article. Review.
Cited by
-
Influence of Fatty Acid Desaturase Enzyme-1 Gene (FADS-1) Polymorphism on Serum Polyunsaturated Fatty Acids Levels, Desaturase Enzymes, Lipid Profile, and Glycemic Control Parameters in Newly Diagnosed Diabetic Mellitus Patients.Int J Mol Sci. 2025 Apr 24;26(9):4015. doi: 10.3390/ijms26094015. Int J Mol Sci. 2025. PMID: 40362254 Free PMC article.
-
The deregulation of arachidonic acid metabolism in ovarian cancer.Front Oncol. 2024 May 2;14:1381894. doi: 10.3389/fonc.2024.1381894. eCollection 2024. Front Oncol. 2024. PMID: 38764576 Free PMC article. Review.
-
Anti-Melanoma Effects of Miconazole: Investigating the Mitochondria Involvement.Int J Mol Sci. 2024 Mar 22;25(7):3589. doi: 10.3390/ijms25073589. Int J Mol Sci. 2024. PMID: 38612401 Free PMC article.
-
Vasorelaxant effects of biochemical constituents of various medicinal plants and their benefits in diabetes.World J Diabetes. 2024 Jun 15;15(6):1122-1141. doi: 10.4239/wjd.v15.i6.1122. World J Diabetes. 2024. PMID: 38983824 Free PMC article. Review.
-
15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects.Open Life Sci. 2025 Apr 29;20(1):20251091. doi: 10.1515/biol-2025-1091. eCollection 2025. Open Life Sci. 2025. PMID: 40321157 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

