Attrition Rates in Multiple Myeloma Treatment under Real World Conditions-An Analysis from the Austrian Myeloma Registry (AMR)
- PMID: 36765918
- PMCID: PMC9913775
- DOI: 10.3390/cancers15030962
Attrition Rates in Multiple Myeloma Treatment under Real World Conditions-An Analysis from the Austrian Myeloma Registry (AMR)
Abstract
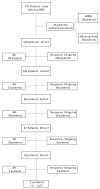
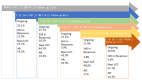
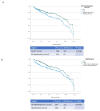
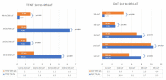
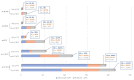
Multiple myeloma (MM) is characterized by serial relapses, necessitating the application of sequential lines of therapy (LoT). Reports on attrition rates (ARs) vary widely. The present study analysed ARs from the Austrian Myeloma Registry. Attrition was defined as being either deceased, progressive without having received another LoT, or lack of follow-up for ≥5 years. A total of 571 patients diagnosed between January 2009 and August 2021 were included (median age: 72 years; median follow-up: 50.8 months). Some 507 patients received at least one LoT. Of the total, 43.6% underwent autologous stem cell transplantation (SCT, transplant eligible = TE)) with primarily VRd (Bortezomib/Lenalidomide/Dexamethasone) given as induction (26.5%), followed by lenalidomide maintenance in 55.7% of cases. Transplant-ineligible (NTE) patients were predominantly treated with Vd (Bortezomib/Dexamethasone, 21.6%), receiving maintenance in 27.1%. A total of 37.5% received a second LoT. ARs across one to five LoTs were 16.7-27%. Frontline induction/ SCT followed by maintenance reduced ARs associated with age and achievement of deep remission in the frontline. Deep remission prolongs follow-up and time-to-next-treatment (TTNT), while high-risk-cyctogenetics negatively affected these outcomes. Our results demonstrate considerably lower ARs for MM patients within the AMR data versus other healthcare systems. Young age and the achievement of significant remissions after optimal frontline therapy resulted in particularly low ARs. These promising results support a key role for the ease of drug access and reimbursement policies in governing long-term MM patient outcomes.
Keywords: attrition rate; multiple myeloma; treatment patterns.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Dimopoulos M.A., Moreau P., Terpos E., Mateos M.V., Zweegman S., Cook G., Delforge M., Hájek R., Schjesvold F., Cavo M., et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. - DOI - PubMed
-
- Bethesda: National Cancer Institute SEER Cancer Stat Facts: Myeloma. [(accessed on 16 January 2022)]; Available online: https://seer.cancer.gov/statfacts/html/mulmy.html.
-
- Fonseca R., Usmani S.Z., Mehra M., Slavcev M., He J., Cote S., Lam A., Ukropec J., Maiese E.M., Nair S., et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20:1087. doi: 10.1186/s12885-020-07503-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

