A Role for Mast Cell-Mediated Antibodies in the Formation of Cholesteatoma and Cholesteatoma-Induced Bone Erosion
- PMID: 36766559
- PMCID: PMC9914080
- DOI: 10.3390/diagnostics13030455
A Role for Mast Cell-Mediated Antibodies in the Formation of Cholesteatoma and Cholesteatoma-Induced Bone Erosion
Abstract
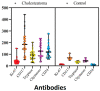
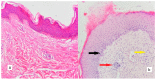
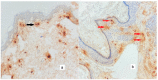
The study aimed to evaluate the effects and relationships between mast cells in the matrix, mast cell enzymes tryptase and chymase, epithelial proliferation, microvascular density, and bone destruction in cholesteatoma. Thirty-five biopsies diagnosed with cholesteatoma and seven healthy skin tissues taken from the retro-auricular region for control were evaluated. Immunohistochemical studies were performed with CD117, CD34, Ki-67, chymase, and tryptase antibodies, in a single session for all cases and the control group. The relationship between erosion size and antibody load was determined. The mean cholesteatoma epithelium Ki-67 was higher than the control group (p < 0.001). CD117-positive mast cells, chymase-positive mast cells, tryptase-positive mast cells, and microvessel density were significantly higher in the cholesteatoma matrix compared to the control group (p < 0.002, p < 0.001, p < 0.005). In the group with bone erosion scores of two and above, immunohistochemical markers tended to be higher. A positive correlation was found between CD117 and chymase, tryptase, and microvessel density; between tryptase, chymase, and microvessel density; and between chymase and microvessel density. CD117-positive mast cells and chymase-positive mast cells stimulate angiogenesis, increase the epithelium's proliferative capacity in the cholesteatoma matrix, and form cholesteatoma. The increased proliferation of cholesteatoma epithelium and increased vascular density in the matrix exacerbate bone erosion.
Keywords: CD117; CD34; Ki67; bone erosion; cholesteatoma; chymase; tryptase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Mast cell phenotype in benign and malignant tumors of the prostate.Pol J Pathol. 2014 Jun;65(2):147-53. doi: 10.5114/pjp.2014.43965. Pol J Pathol. 2014. PMID: 25119176
-
Detection of tryptase-, chymase+ cells in human CD34 bone marrow progenitors.Clin Exp Allergy. 2004 Nov;34(11):1719-24. doi: 10.1111/j.1365-2222.2004.02105.x. Clin Exp Allergy. 2004. PMID: 15544596
-
The increase in tryptase- and chymase-positive mast cells is associated with partial inactivation of chymase and increase in protease inhibitors in basal cell carcinoma.J Eur Acad Dermatol Venereol. 2007 Aug;21(7):908-15. doi: 10.1111/j.1468-3083.2006.02100.x. J Eur Acad Dermatol Venereol. 2007. PMID: 17658999
-
Mast cell chymase: morphofunctional characteristics.Histochem Cell Biol. 2019 Oct;152(4):253-269. doi: 10.1007/s00418-019-01803-6. Epub 2019 Aug 8. Histochem Cell Biol. 2019. PMID: 31392409 Review.
-
Mast cells in vulnerable coronary plaques: potential mechanisms linking mast cell activation to plaque erosion and rupture.Curr Opin Lipidol. 2004 Oct;15(5):567-73. doi: 10.1097/00041433-200410000-00011. Curr Opin Lipidol. 2004. PMID: 15361793 Review.
Cited by
-
Biology of recurrent cholesteatoma in a Romanian young patient - a case report.Rom J Morphol Embryol. 2024 Oct-Dec;65(4):775-780. doi: 10.47162/RJME.65.4.24. Rom J Morphol Embryol. 2024. PMID: 39957039 Free PMC article.
-
Clinical and prognostic effects of microvascular density and FOXP3 positive T cells in breast cancer.Sci Rep. 2024 Dec 5;14(1):30341. doi: 10.1038/s41598-024-82106-2. Sci Rep. 2024. PMID: 39639042 Free PMC article.
References
-
- Yung M., Tono T., Olszewska E., Yamamoto Y., Sudhoff H., Sakagami M., Mulder J., Kojima H., İncesulu A., Trabalzini F., et al. EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma. J. Int. Adv. Otol. 2017;13:1–8. doi: 10.5152/iao.2017.3363. - DOI - PubMed
LinkOut - more resources
Full Text Sources

