Proteoglycan SPOCK1 as a Poor Prognostic Marker Promotes Malignant Progression of Clear Cell Renal Cell Carcinoma via Triggering the Snail/Slug-MMP-2 Axis-Mediated Epithelial-to-Mesenchymal Transition
- PMID: 36766694
- PMCID: PMC9913795
- DOI: 10.3390/cells12030352
Proteoglycan SPOCK1 as a Poor Prognostic Marker Promotes Malignant Progression of Clear Cell Renal Cell Carcinoma via Triggering the Snail/Slug-MMP-2 Axis-Mediated Epithelial-to-Mesenchymal Transition
Abstract
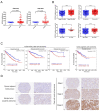
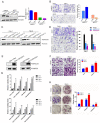
Sparc/osteonectin, cwcv, and kazal-like domains proteoglycan 1 (SPOCK1) has been reported to play an oncogenic role in certain cancer types; however, the role of SPOCK1 in the progression of clear cell renal cell carcinoma (ccRCC) remains elusive. Here, higher SPOCK1 transcript and protein levels were observed in ccRCC tissues compared to normal tissues and correlated with advanced clinical stages, larger tumor sizes, and lymph node and distal metastases. Knockdown and overexpression of SPOCK1 in ccRCC cells led to decreased and increased cell clonogenic and migratory/invasive abilities in vitro as well as lower and higher tumor growth and invasion in vivo, respectively. Mechanistically, the gene set enrichment analysis (GSEA) database was used to identify the gene set of epithelial-to-mesenchymal transition (EMT) pathways enriched in ccRCC samples with high SPOCK1 expression. Further mechanistic investigations revealed that SPOCK1 triggered the Snail/Slug-matrix metalloproteinase (MMP)-2 axis to promote EMT and cell motility. Clinical ccRCC samples revealed SPOCK1 to be an independent prognostic factor for overall survival (OS), and positive correlations of SPOCK1 with MMP-2 and mesenchymal-related gene expression levels were found. We observed that patients with SPOCK1high/MMP2high tumors had the shortest OS times compared to others. In conclusion, our findings reveal that SPOCK1 can serve as a useful biomarker for predicting ccRCC progression and prognosis, and as a promising target for treating ccRCC.
Keywords: SPOCK1; clear cell renal cell carcinoma; epithelial-to-mesenchymal transition; matrix metalloproteinase-2; metastasis; snail/slug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Casuscelli J., Becerra M.F., Manley B.J., Zabor E.C., Reznik E., Redzematovic A., Arcila M.E., Tennenbaum D.M., Ghanaat M., Kashan M., et al. Characterization and Impact of TERT Promoter Region Mutations on Clinical Outcome in Renal Cell Carcinoma. Eur. Urol. Focus. 2019;5:642–649. doi: 10.1016/j.euf.2017.09.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

