Calnexin, More Than Just a Molecular Chaperone
- PMID: 36766745
- PMCID: PMC9913998
- DOI: 10.3390/cells12030403
Calnexin, More Than Just a Molecular Chaperone
Abstract
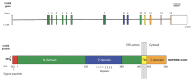
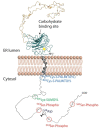
Calnexin is a type I integral endoplasmic reticulum (ER) membrane protein with an N-terminal domain that resides in the lumen of the ER and a C-terminal domain that extends into the cytosol. Calnexin is commonly referred to as a molecular chaperone involved in the folding and quality control of membrane-associated and secreted proteins, a function that is attributed to its ER- localized domain with a structure that bears a strong resemblance to another luminal ER chaperone and Ca2+-binding protein known as calreticulin. Studies have discovered that the cytosolic C-terminal domain of calnexin undergoes distinct post-translational modifications and interacts with a variety of proteins. Here, we discuss recent findings and hypothesize that the post-translational modifications of the calnexin C-terminal domain and its interaction with specific cytosolic proteins play a role in coordinating ER functions with events taking place in the cytosol and other cellular compartments.
Keywords: calcium binding protein; cell signaling; endoplasmic reticulum; molecular chaperone; protein–protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

