Impact of 5-HT6 Receptor Subcellular Localization on Its Signaling and Its Pathophysiological Roles
- PMID: 36766768
- PMCID: PMC9913600
- DOI: 10.3390/cells12030426
Impact of 5-HT6 Receptor Subcellular Localization on Its Signaling and Its Pathophysiological Roles
Abstract
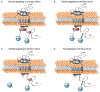
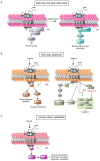
The serotonin (5-HT)6 receptor still raises particular interest given its unique spatio-temporal pattern of expression among the serotonin receptor subtypes. It is the only serotonin receptor specifically expressed in the central nervous system, where it is detected very early in embryonic life and modulates key neurodevelopmental processes, from neuronal migration to brain circuit refinement. Its predominant localization in the primary cilium of neurons and astrocytes is also unique among the serotonin receptor subtypes. Consistent with the high expression levels of the 5-HT6 receptor in brain regions involved in the control of cognitive processes, it is now well-established that the pharmacological inhibition of the receptor induces pro-cognitive effects in several paradigms of cognitive impairment in rodents, including models of neurodevelopmental psychiatric disorders and neurodegenerative diseases. The 5-HT6 receptor can engage several signaling pathways in addition to the canonical Gs signaling, but there is still uncertainty surrounding the signaling pathways that underly its modulation of cognition, as well as how the receptor's coupling is dependent on its cellular compartmentation. Here, we describe recent findings showing how the proper subcellular localization of the receptor is achieved, how this peculiar localization determines signaling pathways engaged by the receptor, and their pathophysiological influence.
Keywords: G-protein-coupled receptor; primary cilium; serotonin; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

