T Cell Energy Metabolism Is a Target of Glucocorticoids in Mice, Healthy Humans, and MS Patients
- PMID: 36766792
- PMCID: PMC9914408
- DOI: 10.3390/cells12030450
T Cell Energy Metabolism Is a Target of Glucocorticoids in Mice, Healthy Humans, and MS Patients
Abstract
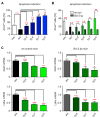
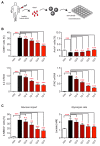
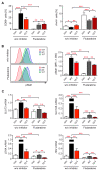
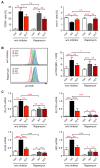
Glucocorticoids (GCs) are used to treat inflammatory disorders such as multiple sclerosis (MS) by exerting prominent activities in T cells including apoptosis induction and suppression of cytokine production. However, little is known about their impact on energy metabolism, although it is widely accepted that this process is a critical rheostat of T cell activity. We thus tested the hypothesis that GCs control genes and processes involved in nutrient transport and glycolysis. Our experiments revealed that escalating doses of dexamethasone (Dex) repressed energy metabolism in murine and human primary T cells. This effect was mediated by the GC receptor and unrelated to both apoptosis induction and Stat1 activity. In contrast, treatment of human T cells with rapamycin abolished the repression of metabolic gene expression by Dex, unveiling mTOR as a critical target of GC action. A similar phenomenon was observed in MS patients after intravenous methylprednisolon (IVMP) pulse therapy. The expression of metabolic genes was reduced in the peripheral blood T cells of most patients 24 h after GC treatment, an effect that correlated with disease activity. Collectively, our results establish the regulation of T cell energy metabolism by GCs as a new immunomodulatory principle.
Keywords: T cells; glucocorticoids; metabolism; multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wüst S., van den Brandt J., Tischner D., Kleiman A., Tuckermann J.P., Gold R., Lühder F., Reichardt H.M. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:8434–8443. - PubMed
-
- Theiss-Suennemann J., Jorss K., Messmann J.J., Reichardt S.D., Montes-Cobos E., Lühder F., Tuckermann J.P., Wolff H.A., Dressel R., Gröne H.J., et al. Glucocorticoids attenuate acute graft-versus-host disease by suppressing the cytotoxic capacity of CD8(+) T cells. J. Pathol. 2015;235:646–655. doi: 10.1002/path.4475. - DOI - PubMed
-
- Baschant U., Frappart L., Rauchhaus U., Bruns L., Reichardt H.M., Kamradt T., Brauer R., Tuckermann J.P. Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc. Natl. Acad. Sci. USA. 2011;108:19317–19322. doi: 10.1073/pnas.1105857108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

