Promotion of Lymphangiogenesis by Targeted Delivery of VEGF-C Improves Diabetic Wound Healing
- PMID: 36766814
- PMCID: PMC9913977
- DOI: 10.3390/cells12030472
Promotion of Lymphangiogenesis by Targeted Delivery of VEGF-C Improves Diabetic Wound Healing
Abstract
Chronic wounds represent a major therapeutic challenge. Lymphatic vessel function is impaired in chronic ulcers but the role of lymphangiogenesis in wound healing has remained unclear. We found that lymphatic vessels are largely absent from chronic human wounds as evaluated in patient biopsies. Excisional wound healing studies were conducted using transgenic mice with or without an increased number of cutaneous lymphatic vessels, as well as antibody-mediated inhibition of lymphangiogenesis. We found that a lack of lymphatic vessels mediated a proinflammatory wound microenvironment and delayed wound closure, and that the VEGF-C/VEGFR3 signaling axis is required for wound lymphangiogenesis. Treatment of diabetic mice (db/db mice) with the F8-VEGF-C fusion protein that targets the alternatively spliced extra domain A (EDA) of fibronectin, expressed in remodeling tissue, promoted wound healing, and potently induced wound lymphangiogenesis. The treatment also reduced tissue inflammation and exerted beneficial effects on the wound microenvironment, including myofibroblast density and collagen deposition. These findings indicate that activating the lymphatic vasculature might represent a new therapeutic strategy for treating chronic non-healing wounds.
Keywords: VEGF-C; diabetes mellitus; inflammation; lymphangiogenesis; wound healing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

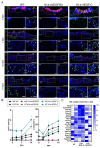
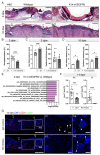
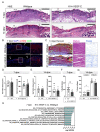




References
-
- Cueni L.N., Detmar M. Lymphatic Vascular System and Lymphangiogenesis. In: Figg W.D., Folkman J., editors. Angiogenesis. Springer; Boston, MA, USA: 2008. pp. 505–516. Chapter 43.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

